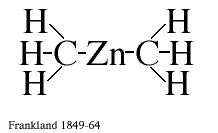Press release

23 October 1973
The Royal Swedish Academy of Sciences has decided to award the 1973 Nobel Prize in Chemistry half each to:
Professor Ernst Otto Fischer, Technical University of Munich, Munich, Federal Republic of Germany and
Professor Geoffrey Wilkinson, Imperial College, London, Great Britain
for their pioneering work performed independently on the chemistry of the organometallic, so called sandwich compounds.
“Chemistry for Chemists”
Chemistry is a scientific discipline with very wide applications ranging from biological-medical to the technological field. In most cases it is therefore easy to indicate potential practical application of discoveries which have motivated a Nobel Prize in chemistry. This year the prize has been awarded for work, practical applications of which are not very obvious, it is a prize in “chemistry for chemists”. A very essential part of a scientific discipline is its structure and its concepts. Fischer and Wilkinson widened the basic concepts of chemistry by their work and therefore also changed the structure of chemistry.
It is necessary to look back in the history of chemistry to be able to appreciate their contributions. The birth of organometallic chemistry can be traced to the 18th century, but the first organometallic compound proper was prepared 1849 by Frankland in Great Britain. This discovery had a great impact on the chemical thinking of the time, mainly because it was the first chemical compound between a metal atom Zn and an organic radical methyl CH3. In the chemical language of our time it was the first compound which contained chemical bonds between a metal atom and carbon atoms (see figure 1). Organometallic compounds of this type have since been prepared and studied in considerable detail. Some of them are well known, or rather ill-known, because of the environmental hazards they have caused, such as organo-mercury and organo-lead compounds. They have, however, also been very useful in preparative chemistry and two Nobel Prizes have been awarded for such applications: 1912 to Grignard and 1963 to Ziegler and Natta. A very remarkable discovery was that the coenzyme vitamin B12 contained direct cobolt-carbon bonds (Nobel Prize to Crowfoot Hodgkin 1964).
Sandwich Compounds
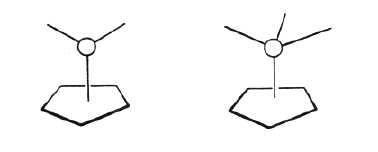
The basic concept of organometallic chemistry was from 1949 and onwards widened through important contributions by the two British chemists Dewar and Chatt and a number of other scientists who investigated compounds where a metal atom was bonded to a bond between two carbon atoms rather than to the individual carbon atoms. Still more striking was, however, the suggestion independently by Fischer and Wilkinson that there existed compounds which contained bonds between metal atoms and a whole molecule, arranged in a way which has not been considered as possible or probable earlier. The first example was the compound called “ferrocen” (see figure 2). The figure shows the structure of this compound in a simplified way. One should imagine carbon atoms placed at the corners of the two plates surrounding the iron atom. The representation of the structure is made in the simplified way to indicate why these compounds have been called sandwich compounds: an iron atom with two plate molecules called cyklopentadienyl groups on each side. This is the most essential part of Fischer’s and Wilkinson’s work: the establishment of the new sandwich compound. They did not prepare the first sandwich but they were the first to grasp the odd nature of the compound and its conceptual importance.
This happened 1952, and during the following ten years they made most of the important contributions in the field. First of all they established the concept by preparing similar compounds with a great number of other metals. Fischer also made a sandwich compound with chromium and benzen molecules (see figure 3), a great surprise for most chemists at that time.
An interesting extension was the preparation of the first open sandwiches (see figure 4). The small molecules which replace the plate molecules on one side of the metal atom were first carbonmonoxide, CO, and later methyl groups like in the first prepared organometallic compound with direct bonds between the metal and carbon atoms. Still more striking was the first preparation by Wilkinson of a sandwich compound containing a direct bond between a metal atom, rhenium, and a hydrogen atom.
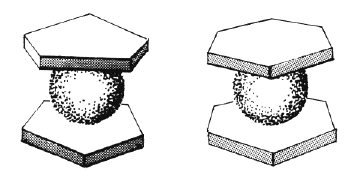 |
| Figure 2 and 3. |
The successful preparations of the methyl and hydrogen compounds led to penetrating international discussions of the reasons for the unexpected stability of these compounds and this discussion has strongly influenced the current ideas of how many organometallic compounds function used as catalysts mainly in reactions involving hydrogen. In this way the study of the sandwich compounds has already been of some importance for the practical applications but a further development can be expected. In Nature no natural compound of sandwich-type is known to exist. They are all made by man, confirming the introductory remark that this is indeed chemistry for chemists. One of the nominators has very clearly defined the achievements of Fischer and Wilkinson and also indicated the relation to future applications: “It is the considered opinion of the nominator that the work of Fischer and Wilkinson has revolutionized transition metal chemistry during the past two decades and played the decisive role in our present day understanding of transition metal-carbon sigma- and pi-bonds and led to major developments in the homogenous catalysis of the reactions of unsaturated hydrocarbons which is also of considerable industrial importance.”
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.