Press release

19 October 1983
The Royal Swedish Academy of Sciences has decided to award the 1983 Nobel Prize for chemistry to
Professor Henry Taube, Stanford University, Stanford, USA,
for his work on the mechanisms of electron transfer reactions, especially in metal complexes.
Chemistry prize awarded to one of the most creative contemporary workers in inorganic chemistry
Chemical reactions were known to man long before chemistry had attained the status of science. It was observed that substances changed their properties under certain external conditions, which is a characteristic of chemical reactions. Thus the ancient Egyptians found that if malachite, a green ore, was fired with charcoal, a red metal was obtained, called copper. It was also found that when clay was baked, ceramic products with properties quite different from clay were obtained.
Much earlier than this, man had found that a piece of dry wood caught fire if it could be made hot enough: changes in the properties of substances occurred only on certain conditions. Temperature was early the factor which was varied in order to bring about changes, and it was also found at an early stage that the speed with which the changes occurred frequently depended on the temperature. With the discovery of black powder it was also noted that processes could take place very rapidly, leading to explosions. The branch of chemistry concerned with how fast chemical reactions take place is known as chemical kinetics, and the scientist engaged in explaining how is said to study the mechanism of chemical reactions.
Millennia of hypotheses, experiments and observations, new hypotheses and new experiments and observations were to pass before a fairly firm scientific structure had been created. At the beginning of this century, progress had been considerable. In particular, a physical-mathematical description of the reactions had been produced, and it was possible in figures and formulas to express the conditions determining whether a chemical reaction would occur, and it was possible to provide mathematical equations for how rapidly it took place. A beginning had also been made in the treatment of reactions which did not pass completely in one direction, as opposed to those mentioned above. It was realized that chemical equilibria existed, and it was possible to deal with these theoretically. It is a characteristic of chemical equilibria that the reacting ions or molecules, although on average bound to another a given bond is not permanent and that the bonds are always being broken down and restored. Three major types of equilibrium reactions have come to be of dominant importance in chemistry. The concepts of acid and base were combined in the acid/base reactions and the pH associated with this.
Metal ions dissolved in water may attract ions or molecules. This is known as complex formation and usually, although not always, occurs as an equilibrium reaction. Finally the combustion of the burning piece of wood and the production of metallic copper from its ore through a reaction with charcoal have been generalized as oxidation and reduction. As a further generalization it has been found that oxidation and reduction are associated with a transfer of electrons, e.g. in metal ions such as cobalt and chromium. Under certain conditions it is possible to make cobalt with three positive charges react with chromium having two positive charges, where cobalt gets only two but chromium three positive charges. The effect is thus that an electron having a negative charge has been transferred from the two-valent chromium to the three-valent cobalt. This is particularly frequent phenomenon in complex compounds of metal ions. Taube has today been awarded the 1983 Nobel Prize for his studies of the mechanisms of electron transfer in metal complexes. Better than anyone else he has helped us understand how these electron transfers take place. It is particularly the structural preconditions governing electron transfers in metal complexes which he has studied. The electron transfer process as such is a separate major problem in theoretical chemistry and physics, where other scientists have contributed more than Taube.
What are the experiments made by Henry Taube and what conclusions has he been able to draw? In his studies, he started from the fact that three-valent ions of cobalt and chromium do not form equilibrium complexes (an example of the exceptions already referred to). The ions or molecules which are bound to these metal ions are therefore joined to them without ever leaving them. But the corresponding two-valent ions form equilibrium complexes. If an ion or molecule bound to the three-valent ion (in this instance, three-valent cobalt) could somehow be marked so that it is possible to find experimentally whether this marked ion or molecule in the electron transfer has at the same time been transferred to the other metal ion (in this instance, two-valent chromium), that is, in the opposite direction as the electron in this case. This was exactly what Taube found, and from this he drew the conclusion that before the electron transfer could take place, a bridge was formed between the metal ions of the ion or molecule which changed places. He proved this in a large number of cases and investigated how the electron transfer was affected by changes in the bridging molecule.
His next step was to lengthen the bridge between the metal ions (while using molecules which could bind two metal ions) and he found that in some instances there was still an electron transfer in spite of the greater distance between the metal ions. There was thus a form of what Taube calls ”distant attack”.
A logical continuation was the bonding of three-valent ions to the two ends of the bridge before reducing this complex with a two-valent ion (in this instance, europium). This reacted rapidly with one of the metal ions and Taube could then follow the slow transfer within the complex (in this case from ruthenium to cobalt) free from all assumptions on how rapidly the bridge was formed.
Finally Taube let the three-valent metal ions on either side of the bridge be identical and could then study if in reduction with an electron this was captured by one of the identical metal ions or it belonged to both, a phenomenon known as delocalization. (Delocalization generally gives rise to strong colours, such as in Prussian blue.)
This entire development was dominated both experimentally and theoretically by Taube, who according to one of the nominations has in eighteen listed instances been first with major discoveries in the entire field of chemistry. The examples selected here, which are all included in the prize award, may seem rather specialized, not to say esoteric. However, during the last ten years it has become increasingly apparent that Taube’s ideas have a considerable applicability, particularly in biochemistry. All respiration which is associated with oxygen consumption is thus also associated with electron transfers, and a growing number of scientists in this field are basing their work on Taube’s concepts of electron transfers in metal complexes.
It should be added that, as already pointed out, Taube has made major contributions throughout the chemistry of complexes. Thus he was the first to produce a complex between a three-valent metal ion, which was based on the ideas developed by Taube in his electron transfer studies.
Finally a quotation from one of Nobel Committee’s reports on Taube: ” There is no doubt that Henry Taube is one of the most creative research workers of our age in the field of coordination chemistry throughout its extent. He has for thirty years been at the leading edge of research in several fields and has had a decisive influence on developments.”
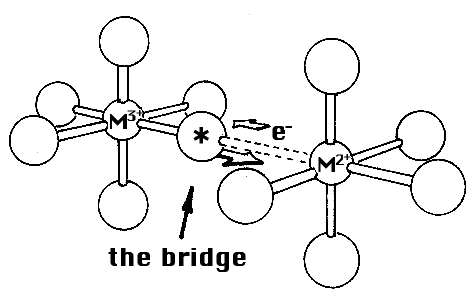
Figure: the bridge.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
