Press release

16 October 1985
The Royal Swedish Academy of Sciences has decided to award the 1985 Nobel Prize in chemistry jointly to
Professor Herbert A. Hauptman, The Medical Foundation of Buffalo, USA, and to
Professor Jerome Karle, US Naval Research Laboratory, USA,
for their outstanding achievements in the development of direct methods for the determination of crystal structures.
Summary
This year’s Nobel Prize in Chemistry has been awarded to Herbert A. Hauptman and Jerome Karle “for their outstanding achievements in the development of direct methods for the determination of crystal structures”. The prize is being awarded for a methodology because of the great importance of this methodology for chemical research. Through Hauptman’s and Karle’s fundamental achievements, the methods have been developed into practical instruments for determining the structure of molecules within both inorganic and organic chemistry – not least within the chemistry of natural products.
The determination of structure involves generating a three-dimensional picture of the positions of the atoms. The picture maps the electron density within the crystal the density is greatest at the centre of the atoms. It can never be less than zero anywhere, and this is the fact upon which the Hauptman-Karle method is based.
Structure determination employs radiation of so short a wavelength that it becomes possible to “see” the atoms – X-rays are normally used for this. This means that the wavelength must be shorter than the distance between each atom. X-rays striking a crystal are deflected and concentrated in different directions, and the intensity of the deflected rays is measured. In order to determine the positions of the atom in a crystal, however, is it not enough to know the direction and intensity of the rays, it is also necessary to know the “phase” of each deflected ray, that is, how much the waves in the different rays are displaced in relation to each other.
The fact that electron density is positive (electrons either exist or they do not) limits the possibilities for phase displacement. Hauptman and Karle have constructed systems of equations that are based on the values of the intensities measured and that describe the limitations. The two scientists have also developed a procedure for solving the equations: the solutions give direct connections between the phases sought.
Since the validity of each equation is only statistically probable, it is necessary to make a large number of measurements and to obtain many times more equations than the number of unknowns to be determined. While this makes the determinations of phase more reliable, it entails comprehensive calculations of the kind that are now feasible using modern computer techniques.
The method is termed “direct” because of the fact that, in contrast with other methods, it gives the structure directly from the data collected.
In order to understand the nature of chemical bonds, the function of molecules in biological contexts and the mechanism and dynamics of reactions, knowledge of the exact molecular structure is absolutely necessary
Background Information
This year’s Nobel Prizewinners in Chemistry, Herbert A. Hauptman and Jerome Karle, have developed what are termed “direct methods” for the determination of crystal structure. This development of a method merits a Nobel Prize since the method now plays an increasingly important role in chemical research. It is therefore of importance to consider the method first.
As early as the turn of the century, chemists possessed a good understanding of the geometrical arrangement of the atoms in carbon compounds. But it is only through structure determination using X-ray crystallography that we have been able to obtain a detailed picture of the distances between the atoms and of the angles between the various bonds. Spectroscopy and electron diffraction have played a complementary role, especially in the case of simpler molecules.
Until the 1960’s it was determination of the arrangement of the atoms that gave the most important new results. The whole of inorganic chemistry was revolutionized, hitherto completely unknown principles of structure being elucidated. Important progress was also made in natural product chemistry. A series of Nobel Prizes describes this development: von Laue in 1914, the Braggs, father and son 1915, Pauling 1954, Perutz and Kendrew 1962, Crick, Watson and Wilkens 1962, Hodgkin 1964, Barton and Hassel 1969, Lipscomb 1976 and Klug 1982.
Lipscomb combined structure determination with far-reaching studies of the nature of chemical bonds, and it is precisely this theory of chemical bonding that requires knowledge of the exact structure of molecules – in other words, accurate bond distances and accurate bond angles.
The need for exact knowledge of structure is great within two areas of chemistry. One of these areas concerns structural problems, especially those associated with the function of molecules in biological contexts. Here, a large number of processes are considered in similar ways under the heading “signal – receptor processes”. Examples of these processes are enzyme activity, antigen – antibody and scent substance – scent receptor. For understanding these signal-receptor processes it is necessary to gain as detailed a knowledge as possible of both signal molecules and receptor molecules (active site). The signal molecules are relatively small and their structure can be determined. The structure of the receptor molecule can also be perceived by analogy with low-molecular compounds. Where giant molecules are involved, structure determination of the type for which Perutz and Kendrew received a Nobel Prize is required. For determining the low-molecular signal molecules the Hauptman-Karle direct method must be used.
In the other important area, the mechanism and chemical dynamics of reactions are studied. Questions being asked also by chemists working with organic synthesis are, for instance: How, at molecular level, does a chemical reaction take place? How does a molecule move, and how is the structure changed in chemical reactions? The most important answers are coming from researchers within theoretical chemistry, but these must in turn have accurate knowledge of the structures of reacting molecules.
To summarize: the last fifteen years have seen a large increase in structure determinations accomplished within both inorganic and organic chemistry, including natural product chemistry. These determinations have been carried out predominantly using “direct methods”. Looking into the future we can predict a further increased need for structure determinations of this kind.
While it is easy to explain the importance for chemistry of the two prizewinners’ development of the methods, it is considerably more difficult without recourse to mathematical formulae to describe the achievement itself in a way that is easy to understand.
When X-rays strike a crystal, they will be deflected only in certain definite directions, where the intensity of irradiation may be measured. To determine the arrangement of atoms in a crystal, however, it is not enough to know the direction and intensity. The “phase” of each ray so deflected must also be known. In special cases, it has been possible to solve this “phase problem” by making use of the fact that “heavy” atoms containing many electrons spread the X-rays more strongly than “light” atoms do. This property of heavy atoms is used both in “Patterson methodology”, which has been very important in structural inorganic chemistry, and in “isomorph substition”. The latter is used when determining the structure of giant molecules such as proteins. In this case the heavy atoms can be bound to the protein without its structure being appreciably altered. This however is not possible for the large number of compounds.
Two facts have created the conditions for the development of the “direct” methods. The first is that electron density, which diffuses the X-rays, can never be negative. The other is that the number of measurements is much greater than the number of equations to be solved, which permits the use of statistical methods. In work done between 1950 and 1956, Hauptman and Karle laid the foundations for a rational exploitation of these possibilities, specially the use of inequalities.
The immense importance of this work for subsequent development may easily be followed in the literature. This is not to say that Hauptman and Karle alone are responsible for the development, and other names must be mentioned in particular. Before Hauptman and Karle published their work, D. Harker and J.S. Kasper proposed the use of one inequality, which represents a special case in the Hauptman-Karle system, and determined a complicated structure using it. Important conceptual contributions were also made by D. Sayre, who anticipated the practical approach which has later come to be used. Isabel Karle´s and M. Woolfson’s contributions to the practical utilization of direct methods have been crucial, and in this connection the development of fast computers has been a prerequisite for the full realization of the value of the method.
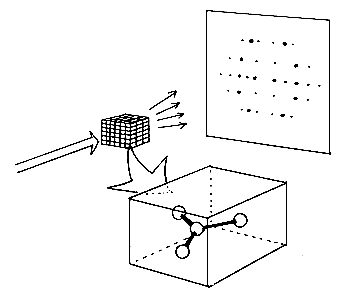
X-rays strike a crystal. The crystal contains a molecule, which is repeated throughout the whole crystal in all directions. The crystal deflects the x-rays in certain definite directions so that the radiation can be seen as spots of different intensity such as in a photographic film. To determine the structure the “phase” of each ray that is deflected must also be known. This determination can be carried out by using the “direct methods”.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
