Press release

15 October 1986
The Royal Swedish Academy of Sciences has decided to award the 1986 Nobel Prize in chemistry jointly to
Professor Dudley R. Herschbach, Harvard University, Cambridge, USA,
Professor Yuan T. Lee, University of California, Berkeley, USA and
Professor John C. Polanyi, University of Toronto, Toronto, Canada
for their contributions concerning the dynamics of chemical elementary processes.
The dynamics of chemical reactions – a fascinating new field of research
Summary
This year’s Nobel Prize in Chemistry has been awarded to Dudley R. Herschbach, Yuan T. Lee and John C. Polanyi for their contributions concerning the dynamics of chemical elementary processes. Their research has been of great importance for the development of a new field of research in chemistry – reaction dynamics – and has provided a much more detailed understanding of how chemical reactions take place.
Dudley R. Herschbach has developed the method of crossed molecular beams, directed and well-defined fluxes of molecules, to and beyond the point where detailed studies of chemical reactions have been possible. He has also elucidated the dynamics of the basic types of reaction. Yuan T. Lee, who initially worked in cooperation with Herschbach, has developed the method of crossed molecular beams further towards its use for general reactions. Most notably, he has used this method for the study of important reactions for relatively large molecules. John C. Polanyi has developed the method of infrared chemiluminescence, in which the extremely weak infrared emission from a newly formed molecule is measured and analysed. He has used this method to elucidate the detailed energy disposal during chemical reactions.
Background
The molecules and atoms in all substances are in perpetual motion, and collisions between the molecules in a gas or a liquid thus occur continuously. When molecules come in close enough contact with each other, redistribution of the atoms can take place between or within them. New molecules form so-called product molecules, which means that a chemical reaction takes place. To effect a reaction, the colliding molecules are often required to have some special property such as high velocity or large internal energy.
The classical description of how chemical reactions occur, and how rates of chemical reaction are measured, belongs to the field of chemical reaction kinetics. This field has developed rapidly during the last few decades, especially regarding experimental methods. The 1967 Nobel Prize in Chemistry was awarded to M. Eigen, Federal Republic of Germany , R.G.W. Norrish and G. Porter, Great Britain, for their studies of extremely fast chemical reactions. In many respects however, fundamental understanding of what molecular features influence the rate of chemical reactions has been slow in developing.
The directions and velocities of the molecular motion in a gas or a liquid are mainly random. Consequently, the collisions between the molecules are ill-defined as regards, for example, the kinetic energy in the collision. The details of the reaction thus become blurred and cannot be observed precisely enough. This problem had not been solved satisfactorily before the development described here.
It was finally possible to solve the problem by using molecular beams formed of directed and spatially well-defined molecular fluxes of low density, often also with well-defined velocities. When two molecular beams are caused to cross each other, the details of the reactions between molecules can be studied. The crossed molecular beam technique is thus one of the most important advances within the field of reaction dynamics.
Dudley R. Herschbach took part in the development of this method almost from the start. His extremely important achievements concerned for example studies of short-lived direct reactions, especially of the two main types, the “rebound” and the “stripping” reaction. He supplemented the commonly-used procedure of detecting the product molecules by deflecting them in magnetic and electric fields, thus circumventing one of the largely-overlooked problems inherent in the early experiments. The discovery of the first long-lived reaction complexes in crossed beams was soon followed by a theoretical description of their formation and decay. The great importance of angular momentum was observed for the first time in these reactions. Subsequent, more extensive studies by Lee, among others, have clearly shown that this type of long-lived reaction is of great general importance.
During this first stage of the development of the field of crossed molecular beams, reactions between alkali atoms and other molecules were almost the only ones which could be studied, due to the method of detection used at that time. Several research groups developed crossed-beam machines for more general reactions. One of the most sophisticated of these was developed at Herschbach’s laboratory, first of all by Yuan T. Lee. This so-called “supermachine” employed two well-defined crossed molecular beams and a moveable mass spectrometric detector, incorporating electron impact ionization and several stages of differential pumping.
Both Lee and Herschbach, as well as other researchers, have used this type of molecular-beam apparatus for detailed studies of a large number of chemical reactions. Lee has led the development towards chemically important reaction systems by investigating reactions between organic molecules and fluorine or oxygen atoms. Short-lived direct reactions as well as longlived reactions have been observed for large systems such as these. This confirms the universal validity of the early results from studies of alkali-containing reaction systems. Extremely important reactions, of immediate significance for combustion chemistry and atmospheric chemistry, have been studied by Lee during recent years.
Another very important method for the detail study of chemical reactions has been developed by Polanyi, the infrared – (IR) – chemiluminescence method. This development took place concurrently with the formation of the crossed molecular beam field. This complementary method resembles the crossed molecular beam method in many respects, but involves measurement and analysis of the extremely weak infrared emission from the product molecules in some chemical reactions. Excess energy from the reaction is deposited as internal energy in the product molecules, which after some delay emit the energy in the form of infrared light. Spectroscopic analysis of this light reveals directly the quantum mechanical states which the product molecules occupied. This gives indirect but extremely important information on the multi-dimensional surface describing the potential energy for the system. The potential energy surface is the fundamental, but in most cases largely unknown factor, which determines the detailed behaviour of a chemical reaction.
Polanyi has to a large extent combined a description of the potential energy surface for the reactions studied with the experimental findings. He has for example described how the existence and location of an energy barrier on the potential energy surface modifies the dynamics of the reaction. Further, he has observed that the product molecules in some cases belong to two different, well separated, classes with respect to the internal energy distribution. The method which he has developed can be considered as a first step towards the present more sophisticated, but also more complicated, laser-based methods for the study of chemical reaction dynamics.
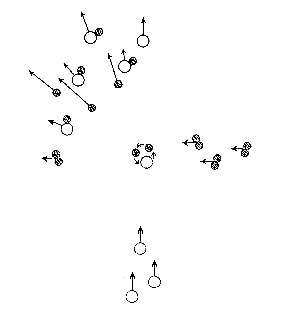
In the figure, two directed molecular fluxes are shown, .i.e. idealized molecular beams. In the crossing region, a reaction can take place and new molecules can form. In this special case oxygen atoms (open circles) react with hydrogen atoms (two filled circles), and form a long-lived complex, which is an energy-rich and thus unstable water molecule. Each complex dissociates finally to a hydrogen atom and a hydroxyl radical. This reaction has been studied in crossed molecular beams as well as with infrared chemiluminescence.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
