Press release

14 October 1987
The Royal Swedish Academy of Sciences has decided to award the 1987 Nobel Prize in chemistry jointly to
Professor Donald J. Cram, University of California, Los Angeles, USA, to
Professor Jean-Marie Lehn, Université Louis Pasteur, Strasbourg, and College de France, Paris, France, and to
former research chemist Charles J. Pedersen, Du Pont, Wilmington, Delaware, USA
for their development and use of molecules with structure-specific interactions of high selectivity.
Awarded for syntheses of molecules that mimic important biological processes
Summary
This year’s Nobel Prize in Chemistry has been awarded to Donald J. Cram, USA, Jean-Marie Lehn, France and Charles J. Pedersen, USA for their development and application of molecules with highly selective structurespecific interaction, i.e. molecules that can “recognize” each other and choose with which other molecules they will form complexes. The laureates have been rewarded for synthesising organic compounds of low molecular weight and with very special properties. The molecules in these compounds are designed principally to bind cations (positive ions), but also anions (negative ions) and neutral-molecules, in a specific and selective manner. The three researchers have studied chemical and physical properties of these complexes and have elucidated the factors that determine the ability of the molecules to recognize each other and fit into one another like a key fits a lock.
Molecules have been produced that mimic the mode of action of enzymes. The laureates’ research has been of great importance for developments within coordination chemistry, organic synthesis, analytical chemistry and bioorganic and bioinorganic chemistry, and has thus laid the foundation for the active interdisciplinary area of research within chemistry that has now come to be termed host-guest chemistry or supramolecular chemistry.
Background Information
At the basis of many biological processes lies the ability of molecules to recognize each other and to form well-defined complexes. Well-known examples are substrates bound to enzymes, signal substances bound to receptors, antibodies bound to antigens and metal ions bound to ionophores. In most cases, one or more compounds of low molecular weight bind to a specific region in a high-molecular-weight compound, most often a protein or a nucleic acid. The binding is very specific and selective and the low molecular-weight compound must fit the high like a key in a lock.
Inorganic chemists have long dreamed of synthesising relatively uncomplicated organic compounds that perform the same functions as natural proteins. Great progress towards this goal has been made over the last 20 years, and it is the pioneering achievements in this particular area that are now being recognized.
In 1967, Charles J. Pedersen published two works, which have now become classics, describing methods of synthesising cyclic polyethers, which he named crown ethers. Pedersen showed that these compounds have remarkable and unexpected properties and that they can even bind the alkali metal ions of lithium, sodium, potassium, rubidium and caesium into complexes in which the lithium ion is the smallest and the caesium ion the largest. He also found that, depending on the structure of the crown ether, potassium could for instance be bound before caesium. Simply expressed, the selectivity is determined by the fact that different crown ethers include “holes” of different sizes, into which different spherical metal ions fit.
By building on Pedersen’s fundamental discovery, Jean-Marie Lehn in 1969 developed bycyclic compounds of crown ether type which he called cryptands and which show even higher selectivity when forming complexes.
Jean-Marie Lehn and Donald J. Cram have subsequently each developed increasingly sophisticated organic compounds which when forming complexes leave fissures and cavities where low-molecular-weight compounds with different types of geometry can be bound. With this work, Pedersen, Lehn and Cram laid the foundations of what is today one of the most active and expanding fields of chemical research, a field for which Cram has coined the term host-guest chemistry while Lehn calls it supramolecular chemistry.
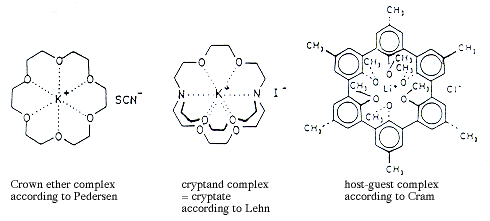
Figure 1.
Cram in particular, using advanced organic-sythetic engineering and molecular mechanics calculations, has designed completely immobile host molecules that form particularly strong complexes of extremely high selectivity. Thus, for example, a host molecule with a 420 000-times-stronger tendency to bind sodium ions than to bind lithium ions has been synthesised. In addition to these alkali ions and other metal ions, it has been possible to produce host molecules that bind organic positive ions (cations such as diazonium and alkylammonium ions), as well as other host molecules which can bind small neutral molecules or negative ions (anions such as phosphate ions and organic carboxylate). Through their detailed investigations of the structures, physical properties and chemical reactions of the complexes, Lehn and Cram have increased our understanding of the factors determining the structure-specific interaction of high selectivity.
These examinations have also contributed to our understanding of ion transport via biological membranes. Selective cation binding has already found many applications. Using different types of host molecule it is possible, for example, to extract radioactive strontium or toxic cadmium and lead ions without affecting other ions, which is very interesting in terms of protection of the environment. Such high selectivity has been achieved that it is even possible to separate isotopes of the same element. Within analytical chemistry, selective complex formation has led to the development of ion-selective electrodes and other types of cation sensor. Certain transition metal complexes also show catalytic activity in photochemical processes, for example the photochemical decomposition of water to hydrogen, which may be of significance in energy production.
This formation of complexes is also being applied increasingly in organic synthesis, not least through Cram’s success in producing crown ethers that help in separating the mirror images of aminoacids.
The goal is to produce synthetic host molecules that recognize biologically active molecules. Thus Lehn has produced a host molecule for the signal substance acetylcholine, which is so important in humans and animals.
The explosive development of the art of organic synthesis has enabled Cram and Lehn to produce hosts that to some extent mimic enzymes such as proteases, ATP-ases and transacylases (see Fig. 2). Supercomplexes which bind organic substrates and metal ions have recently been produced by Lehn (see Fig. 3). It will thus be possible to produce supermolecules which do not suffer from the present limitations on substrate structure and reaction type in, for example, enzymes. Through their work, Cram, Lehn and Pedersen have shown the way.
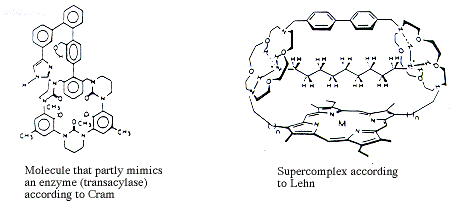 |
| Figure 2 and 3. |
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
