Press release

19 October 1988
The Royal Swedish Academy of Sciences has decided to award the 1988 Nobel Prize in chemistry jointly to
Dr. Johann Deisenhofer, Howard Hughes Medical Institute, Dallas, Texas, USA (German citizen) Professor Robert Huber, Max-Planck-Institut für Biochemie, Martinsried, Federal Republic of Germany and Dr. Hartmut Michel, Max-Planck-Institut für Biophysik, Frankfurt/Main, Federal Republic of Germany,
for the determination of the three-dimensional structure of a photosynthetic reaction centre.
Photosynthesis – the most important chemical reaction on earth
Summary
This year’s Nobel Prize in Chemistry, has been awarded to Johann Deisenhofer, Robert Huber and Hartmut Michel. They are the first to succeed in unravelling the full details of how a membrane-bound protein is built up, revealing the structure of the molecule atom by atom. The protein is taken from a bacterium which, like green plants and algae, uses light energy from the sun to build organic substances. All our nourishment has its origin in this process, which is called photosynthesis and which is a condition for all life on earth.
The organic substances serve as nourishment for both plants and animals. Using the oxygen in the air, they consume these nutrients through what is termed cellular respiration. The conversion of energy in photosynthesis and cellular respiration takes place through transport of electrons via a series of proteins, which are bound in special membranes. These membrane-bound proteins are difficult to obtain in a crystalline form that makes it possible to determine their structure, but in 1982 Hartmut Michel succeeded in doing this. Determination of the structure was then carried out in collaboration with Johann Deisenhofer and Robert Huber between 1982 and 1985.
Photosynthesis in bacteria is simpler than in algae and higher plants, but the work now rewarded has led to increased understanding of photosynthesis in these organisms as well. Broader insights have also been achieved into the problem how electrons can, at an enormously high speed (in a billionth – 10-12 – of a second), be transferred in biological systems.
Background
Photosynthesis is the most important chemical reaction in the biosphere, as it is the prerequisite for all higher life on earth. In this process light from the sun is converted into chemical energy, which is used as nutrition not only by the photosynthetic organisms themselves but also by animals which eat such organisms (e.g. cows eating grass), by other living beings (e.g. humans) eating these animals, and so on through the nutritional chain.
The energy necessary for life processes is to a large extent liberated in the combustion of carbohydrate and fats by the oxygen of the air in cellular respiration. This can, however, continue indefinitely only because the nutritional substances consumed are re-made in the photosynthesis of green plants. Here the plants build up, with aid of solar energy, complicated organic compounds from two simple inorganic molecules, carbon dioxide and water, with the concomitant liberation of oxygen. Photosynthesis and respiration thus have as a result that the sun drives a continuous cyclic process in the biosphere:
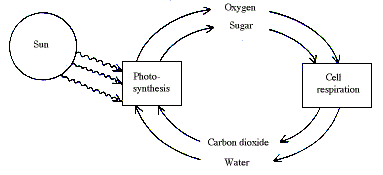
A simpler form of photosynthesis, which leads to the formation of organic material without liberation of oxygen, is found in certain bacteria.
Photosynthesis and respiration comprise electron transfer between proteins, which often contain metal ions, e.g. iron, in specific electron-transport chains. The principles for electron transfer between simple metallic compounds have been analyzed in detail by the Nobel Prize winner for chemistry in 1983, Henry Taube. An important goal in the chemical research of today is to extend these contributions in order to explain electron transfer between the more complicated biochemical molecules.
The electron-transport proteins in photosynthesis as well as in respiration are organized as complicated molecular aggregates bound to membrane systems of two specific cell organelles, chloroplasts and mitochondria. The energy liberated during the electron transport is used to pump protons across the membranes, so that a difference in pH and electrical potential between the two sides is created. This electrochemical potential is then used to drive the synthesis of adenosine triphosphate (ATP), the universal energy storage molecule in living cells, according to the chemiosmotic mechanism formulated by the British biochemist Peter Mitchell (Nobel Prize for chemistry 1978).
Membrane-bound proteins are difficult to obtain in solution and to purify to a form allowing a determination of their detailed structure in three dimensions. Before 1984 there were only fuzzy structural pictures of two membrane proteins available. These pictures had been obtained by an electron microscope technique developed by Aaron Klug, the Nobel Prize winner for chemistry in 1982. The situation changed drastically, however, in 1982, when Hartmut Michel in systematic experiments succeeded in preparing highly ordered crystals of a photosynthetic reaction centre from a purple bacterium, allowing the determination of the structure in atomic detail. The structural work was performed in the period 1982-85 in collaboration with Johann Deisenhofer and Robert Huber.
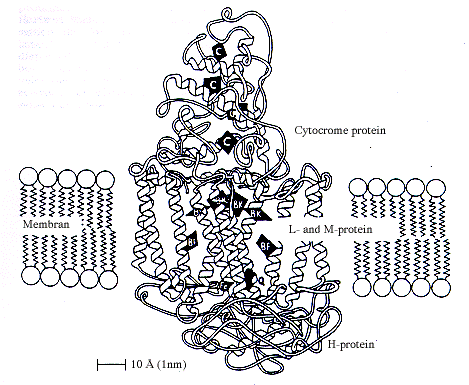 |
| Schematic illustration of a reaction centre in a membrane in a photosynthesising bacterium. The figure shows how the photosynthetically active components bacteriochlorophyll (BK), bacteriopheophytin (BF), quinone (Q) and iron (Fe) and the haemgroups of the cytocrome are arranged in the four proteins forming the reaction centre. |
As already mentioned, the photosynthetic apparatus in bacteria is simpler than in algae and higher plants. The structural work has, however, shown that there is a close relationship between the bacterial reaction centre and the oxygen-evolving protein complex in higher plants, so that the structure determined can be used also to increase our understanding of photosynthesis in general. The structural picture agrees well with the order of the electron transfer steps established earlier by more indirect methods. The detailed structure now forms the basis for more precise attempts to explain theoretically the course of the individual chemical steps.
The structural determination rewarded has considerable chemical importance far beyond the field of photosynthesis. Many central biological functions in addition to photosynthesis and cell respiration are associated with membrane-bound proteins. Examples are transport of chemical substances between cells, hormone action and nerve impulses. The structure of the reaction centre has clarified the principles governing the three dimensional structure of proteins spanning biological membranes, e.g. ion pumps and other transport proteins. Thanks to the method of crystallization developed by Hartmut Michel the prospects of obtaining detailed structural information also for other membrane proteins have improved. Not least important is the fact that the reaction centre structure is an indispensable tool in the attempts of theoretical chemists to understand how electron transfer in biological systems can occur with very high velocities (even in one billionth [U.S., trillionth] of a second) across large distances (more than ten intervening atoms) on the molecular scale.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
