To build “left- or right handed” molecules
Many organic molecules are three-dimensional and can exist in two mirror image forms, one left handed and one right handed. A synthesis gives, in general, equal amounts of the two mirror image forms of the molecule. These might have completely different biological activity. Natural products, i.e. chemicals found in Nature, are usually either “left- or right handed”.
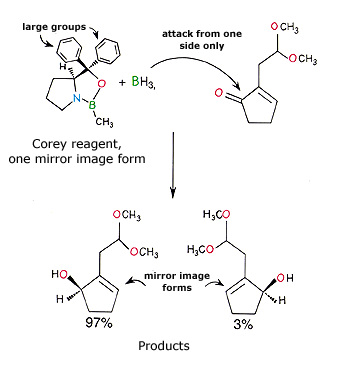
E.J. Corey has contributed to the development of new methods in asymmetric synthesis, which give only one of the mirror image forms. In the reaction shown below only one of the mirror image forms of a Corey-reagent is used. It contains an important nitrogen-boron bond. The reagent is first reacted with borane (BH3) which can then reduce a carbonyl group ![]() to an alcohol
to an alcohol ![]() . The reagent contains very large groups which allow it to reach the carbonyl group from one side only (from above as drawn). Thus, one of the two mirror image forms is formed selectively.
. The reagent contains very large groups which allow it to reach the carbonyl group from one side only (from above as drawn). Thus, one of the two mirror image forms is formed selectively.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
