George A. Olah
Article

Hydrocarbons for the 21st century – The work of the Loker Hydrocarbon Research Institute
by George A. Olah
1994 Nobel Prize laureate in chemistry
This article was published on 6 September 1999.
Hydrocarbons derived from petroleum, natural gas, or coal are essential in many ways to modern life and its quality. The bulk of the world’s hydrocarbons is used for fuels, electrical power generation, and heating. The chemical, petrochemical, plastics and rubber industries are also dependent upon hydrocarbons as raw materials for their products. Indeed, most industrially significant synthetic chemicals are derived from petroleum sources. The overall oil use of the world now exceeds ten million metric tons a day. Ever increasing world population (about 6 billion to increase to 10 billion in a few decades) and energy consumption and finite non-renewable fossil fuel resources, which are going to be increasingly depleted, are clearly on a collision course. New solutions will be needed for the 21st century if we are to maintain the standard of living the industrialized world has gotten used to and the developing world is striving to achieve.
Recognizing the need for a long-range program of basic research and graduate education in the field of hydrocarbon chemistry, the University of Southern California established its “Loker Hydrocarbon Research Institute”1 in 1977. Generous donations from Donald and Katherine Loker, as well as other friends and supporters helped build an outstanding facility and program.
Hydrocarbon chemistry
Hydrocarbons, the principal compounds of oil and natural gas, have to be chemically altered to make useful products and materials. This is carried out by chemical and petrochemical industries in processes such as isomerization, alkylation homologation, etc. These processes are frequently catalyzed by acids and involve electron deficient intermediates called carbocations. The Loker Institute has pioneered new methods to study such processes and their mechanisms. Research is also aimed at more efficient utilization of fossil fuel resources including recycling of carbon dioxide (a greenhouse gas) to useful materials. Studies are also directed towards developing new synthetic methodologies for chemical bond making and bond breaking processes. Polymeric materials derived from simple hydrocarbon precursors are the basis for new materials with exceptional electrical, optical, and magnetic properties. These materials find applications in information technology, photochemical energy conversion and biomedical devices.
Carbocarbons and their chemistry
In studying hydrocarbons and their conversions, a wide variety of highly acidic systems called superacids have been developed. When higher valent Lewis acid fluorides such as SbF5 and TaF5 are combined with Brönsted acids such as HF or FSO3H, acids many billions of times stronger than sulfuric acid are obtained. In such superacidic media the lifetime of carbocations are sufficiently long to be examined by a variety of chemical and physical methods including nuclear magnetic resonance spectrometry.
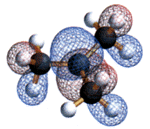
Tertiary Butyl Cation
Copyright © Loker Hydrocarbon Research Institute
Acid catalyzed conversion of hydrocarbons such as cracking, isomerization, alkylation, oligo- and poly-condensation, etc. are of substantial importance. The fundamental chemistry of such hydrocarbon conversions involves carbocations and their reactions. Novel environmentally benign acid systems, including solid acids, are developed to overcome difficulties connected with toxic acids such as hydrofluoric or sulfuric acid. Isomerization and alkylation of saturated hydrocarbons to provide high octane gasoline are of particularly great importance in the petroleum industry. The Loker Institute has developed an environmentally friendly and practical alkylation process for the manufacture of high octane gasoline by using a modified hydrogen fluoride catalyst system of greatly reduced volatility and toxicity.

Dr. Olah preparing t-Butyl Cation.
Photo by Dr. Herwig Buchholz
In addition, the use of superacidic catalysts allow new ways to hydro-treat coals, shale oil, tar sands and other heavy petroleum sources and residues, and yield liquid hydrocarbons. New and environmentally safe gasoline and diesel fuel additives were also developed, resulting in higher octane gasoline and higher octane diesel fuels. These additives have also resulted in cleaner burning fuels and opened the way to exclude currently used other toxic additives.
Conversion of methane or carbon dioxide to hydrocarbons
The direct conversion of methane (i.e. natural gas) to higher hydrocarbons and derived products offers a viable alternative to Fischer-Tropsch chemistry (utilizing synthesis gas, i.e. CO and H2). Until recently, the utilization of methane as a chemical building block was limited to free radical reactions (combustion, nitration, chlorination, etc.). Various stoichiometric organometallic insertion reactions were also discovered, but their use is so far not practical. Superacid catalysts developed at the Institute permit oxidative condensation of methane to higher hydrocarbons, as well as the selective electrophilic conversion of methane to its mono-substituted derivatives such as methyl halides and methyl alcohol. Monosubstituted methanes can be further condensed to ethylene, propylene and derived hydrocarbons over zeolites or bifunctional acidic-basic catalysts, giving access to a whole range of hydrocarbons essential to our everyday life.
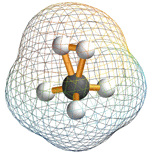
Methonium ion
Copyright © Loker Hyrdocarbon Research Institute
Mechanistic aspects of the methane conversion chemistry, particularly the role of pentacoordinate CH5+-type carbocationic intermediates, were also studied. Kekule’s conclusion dating back to the 1860’s that carbon cannot bound to more than four atoms of groups, i.e. it cannot exceed tetravalency, was refuted by discoveries obtained at the Institute. Dr. Olah’s substantial body of work in this area resulted in the realization that in electrodeficient (carbocationic) systems carbon can coordinate with five, six or even seven atoms or groups simultaneously and laid the foundation to what is now recognized as hypercarbon chemistry.
When hydrocarbons are burned they form carbon dioxide and water. They are thus non-renewable on the human time scale. Excessive burning of fossil fuels leads to increased atmospheric levels of carbon dioxide, which has been linked to global warming and climatic changes. In addition to trying to keep carbon dioxide levels down through reducing burning of fossil fuels (the basis of the 1997 Kyoto agreement), new solutions are needed. An innovative new approach pursued by the Institute is directed at reversing the process by producing hydrocarbons from carbon dioxide and water via methyl alcohol. Some of the underlying chemistry to convert carbon dioxide using hydrogen gas (obtained by electrolytically splitting water) is known. Metal or superacid catalyzed reduction pursued by the Institute has made significant progress to bring about the feasibility of CO2 conversion to methanol. However, electricity needed for generating hydrogen is costly and remains the key to practical applications. As we still cannot store electricity efficiently, power plants in their off-peak periods could produce hydrogen as a means of storing electricity. Hydrogen then could be used to recycle CO2 (from smokestack emissions or other concentrated sources, eventually even the atmosphere) into methyl alcohol and derived fuels. The carbon dioxide recycling technology now under development allows us not only to produce useful fuels and hydrocarbon products, at the same time can contribute to mitigating CO2 related global warming.
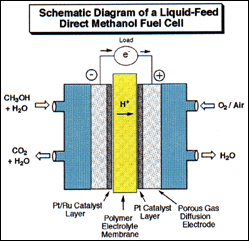
Diagram by Dr. G. K. Surya Prakash
Methyl alcohol and derived fuels can also be used to produce electricity in the new direct oxidation liquid feed fuel cells developed jointly by the Loker Institute and Caltech-JPL. When operating the fuel cell in its “reversed mode”, carbon dioxide and water can be electro-catalytically reduced to methyl alcohol. While the recycling of carbon dioxide into hydrocarbons is a highly energy demanding process some applications, i.e. solar power related applications, may not be overly concerned with this high energy input requirement.
Even if technologies to generate energy from alternate sources are further developed (i.e. atomic, solar, wind, etc.), a concentrated research effort is required to find long-range solutions for future hydrocarbon needs. The effort must include the development of alternative hydrocarbon sources, a search for new chemistry directed towards exploitation of renewable fuels, as well as the development of more efficient and environmentally acceptable ways of utilizing and recycling our present resources.
The final solution to the shortage of hydrocarbons will come only when mankind can produce cheap energy through safer atomic energy (or even fusion) and other alternate sources. With abundant cheap energy, hydrocarbons will be produced from carbon dioxide of the atmosphere and water. In the meantime, however, it is essential that solutions be found that are feasible within the framework of our existing technological base.
Notes
1. The Loker Hydrocarbon Research Institute

The Loker Hydrocarbon building (east elevation)
Photo: Adrian Velicescu
Located at the heart of the University of Southern California campus in Los Angeles, California, the Loker Institute’s 43,000 square feet building features well-equipped state-of-the art laboratories and an attractive work environment. The George and Judy Olah library and the splendid reading room atop the building greatly facilitate the research and educational efforts. The Institute’s plant and equipment represents an investment in excess of twenty-five million dollars. These assets were provided through private donations. At any time about 60 researchers work in the Institute. Their work is ably-supported by a small, but dedicated administrative and technical staff. Financial support for the Institute’s work comes from an annual budget and salaries appropriated by the University, research grants and contracts, income from endowment funds, gifts and donations, as well as income from patents of Institute scientists.
There are four endowed professorships associated with the Institute. The overall annual operational cost of the Institute amounts to about five million dollars. To assure the long-range stability of the Institute, our friends and supporters established endowments, which are augmented continuously. Income from these endowments supplement operating funds, and allows the Institute to carry out exploratory pioneering research. It also makes possible visits and lectures by outstanding national and international scholars and supports scientific symposia hosted by the Institute. An advisory board chaired by Mrs. Katherine Loker, oversees the work at the Institute.

Dr. Olah receiving the 1994 Nobel Prize in Chemistry
© Pressens Bild AB. Photo: Jan Collsiöö
Scientific and Educational Goals
The Loker Institute’s goal is to further fundamental research and advanced training in the broad area of hydrocarbon research. Its work has been guided since its inception by its director, Nobel Laureate Professor George A. Olah.
To date, the Institute’s scientific work has resulted in over 1000 refereed publications in leading technical journals and more than a dozen monographs and books dealing with fundamental research on hydrocarbons. Scores of patents have been issued based on discoveries at the Institute, some of which resulted in industrial processes (vide infra).
A growing need for research in the area of hydrocarbon chemistry also implies an increased demand for chemists who are trained in this field of science. An essential part of the Loker Institute’s mission is to train future generations of scientists by creating and maintaining an educational environment which fosters innovative and practical research in the advanced chemistry of hydrocarbons while helping individuals realize their scientific and academic potential. Since its inception, the Institute has trained more than 300 Ph.D. and post-doctoral fellows who have come from all corners of the world to the Institute. Graduates of the Institute have excelled in both industry and academia. Their success is a testimony to the Institute’s efforts.
First published 6 September 1999
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
