“The Binding Change Mechanism”
|
Paul D. Boyer and John E. Walker have shown how the enzyme ATP synthase makes ATP. ATP synthase is found in chloroplast and mitochondrial membranes and in the cytoplasmic membrane of bacteria. A difference in hydrogen ion concentration across the membrane drives the enzyme to synthesise ATP. Using chemical methods Paul Boyer proposed that ATP synthase is like a cylinder with alternating alpha and beta subunits. An asymmetrical gamma subunit in the middle of the cylinder causes changes in the structure of the beta subunits when it rotates (100 r.p.s.). He termed these structures open (betaO), loose (betaL) and tight (betaT). |
||
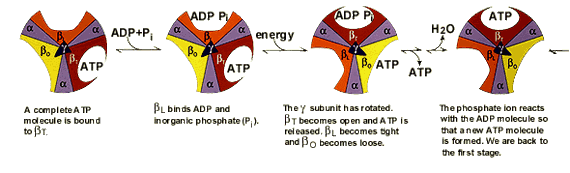 |
||
|
John Walker crystallised the enzyme to study its details. He established that Boyer’s proposal for how ATP synthesis takes place, the “molecular machine”, was correct. |
||
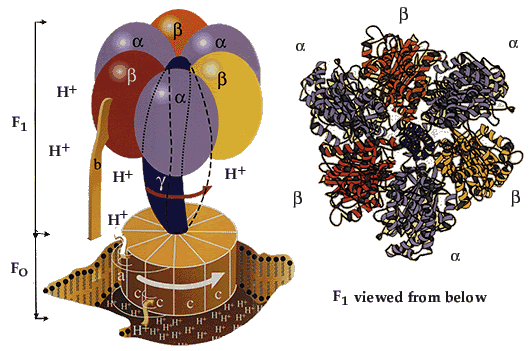 |
||
ATP synthaseThe enzyme consists of an FO part bound in the membrane and a projecting F1 part. The F1 part is known in detail, while less is known about the FO part. The FO part consists of three different protein molecules (subunits a, b and c). When hydrogen ions flow through the membrane via a disc of c subunits, the disc is compelled to rotate. The gamma subunit in the F1 part is fixed to the disc and therefore rotates with it. The alpha and beta subunits in the F1 part, however, cannot rotate because they are locked in a fixed position by the b subunit, which is anchored to subunit a in the membrane. As the gamma subunit functions as an assymmetrical axle, the beta subunits are compelled to undergo the structural changes described in the figures above. |
||
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
