Osamu Shimomura
Biographical
A wartime childhood

I was born on August 27, 1928, in the town of Fukuchiyama, Kyoto-Fu, Japan. My father Chikara was an army captain of the Fukuchiyama regiment. In the spring of 1933, my father took a post in Manchuria, which was under Japanese occupation. Because there were some insurgents in the Manchurian area, my mother Yukie, my younger brother Sadamu, and I chose to stay in Japan and moved to Sasebo, Nagasaki Prefecture, where we lived with my grandmother, Tsuki Shimomura. After the insurgents were wiped out, we moved to Renzankan, Manchuria in March 1935. A sister, Toshiko, was born there, but she died of pneumonia one year after her birth. I remember my mother’s deep sorrow after Toshiko’s death.
In early 1938, my father was transferred to an army position near the Soviet border. The rest of my family moved back to Sasebo. Mother soon departed to Manchuria again to live with my father, and Sadamu and I were in the custody of grandmother Tsuki for about one year. Because I was the elder son, Tsuki raised me carefully and strictly as the heir of the Shimomura family. Since I was physically not very strong, she tried to feed me various nutritious foods like omelet with ground beef and soybean milk. Grandmother was very strict about manners and etiquette. I always had to keep a good posture in her presence. She often said, “the samurai betrays no weakness when starving.” After I bathed, she would check behind my ears and neck for dirt. If she found any, she would say it would be ignominious to be dirty when I was beheaded (it is sometimes honorable for a samurai to commit hara-kiri and then be beheaded). I knew she was talking about the importance of readiness, but it was a little scary. A year later, father took mother back home and stayed only briefly (Fig. 1).

In April 1941, I entered the seventh grade at Sasebo middle school. Japanese militarism was maximal at the time. We had to perform military exercises once or twice a week under the direction of the officers attached to the school. Later that year, my father was transferred to Osaka, and after the Pacific War began on December 8, we joined my father there. At school in Osaka, my teacher happened to be Shizuo Ito, a well-known poet from Isahaya. I contracted tuberculosis and was often tired; though I studied hard, my class rank was poor. I was interested in making model airplanes at the time. Once I built a plane based on an article in a scientific magazine, and I exhibited it at a large department store. My sister, Setsuko, was born, and I had to babysit occasionally.
When I was in the ninth grade, the war became clearly unfavorable to Japan, and my father was sent to Thailand. At school, missing lectures became common and military exercises increased. Once, on an overnight march to Mt. Ikoma, I saw many tiny irregular spots of light on the road. Now I think they were probably luminous earthworms crushed by shoes. In the summer of 1944, my father wrote us to move to the countryside to avoid American bombings. My mother chose to move us to her parents Fujiyama’s house in Isayaha near Nagasaki. That September, on my first day of school (tenth grade), the teacher told us, “This class is mobilized to the Omura Naval Aircraft Arsenal immediately. Other classes are mobilized to Mitsubishi shipyard and arms factory in Nagasaki.” Because the National General Mobilization Law had been activated earlier, I expected to be mobilized sooner or later, but I didn’t think it would be on the first day of school. From that day on, we did not listen to lectures or study at school; we worked.
Two months after I started working at the Omura Naval Aircraft Arsenal, the arsenal was destroyed by bombing. That day, when the air-raid siren sounded, some students took shelter in an underground bunker and the rest of us ran to the edge of the airfield, then waited in a ditch. Soon I saw a formation of more than 20 B-29s coming from the west, and the bombing started. A stray bomb fell nearby and showered us with sand and gravel. The bombing destroyed most of the facilities, but I saw that one hangar was still standing and some people were trying to pull a fighter plane out of it. We rushed there to help them. However, an enemy plane was ahead in the smoke-covered sky. At the moment we reached the hangar, we were showered with numerous incendiary bombs. I heard someone shouting an order to take refuge. We ran between bombs burning with white flame; I saw a person who had been hit on the shoulder running with one arm dangling.
After the air raid, we learned some of the students had died in the underground bunker, and that our dormitory had burned down. Within a month, several wooden factory buildings were built between the hills near Isahaya, and we were ordered to work there. I attended the factory every day even before it was completed; if I found nothing to do I would often lie down in a sweet-potato field nearby and watch large formations of B-29s going east high above Mt. Tara-dake. It was beautiful to see the shining silver B-29s against the background of blue sky. Then, in about 10 minutes, I would see black smoke in the Ohmuta industrial area on the opposite shore of the Ariake Sea, and I could only imagine the scene of carnage over there.
The new factory was for the repair of fighter engines. My job was to smooth the face joining the crank case to the cylinder. My class graduated in March, 1945, at the factory, without a graduation ceremony or diplomas. I was 16 years old. After graduation, the student mobilization continued.
The Nagasaki atomic bomb and the end of the war
On August 6, 1945, news reports informed us that the city of Hiroshima had been completely destroyed by a new type of bomb; we didn’t know what kind. Three days later, shortly before 11 AM, a siren sounded at the Isahaya factory, notifying us of an air raid. As usual, rather than going into a bunker, I went to the top of a nearby hill with a couple of friends and looked at the sky. We saw a single B-29 going from north to south towards Nagasaki, about 15 km away. I thought that its course was unusual. The B-29 dropped two or three parachutes and I heard sporadic gunshots. Watching carefully, I saw no people attached to the parachutes. Within a few minutes, another B-29 followed the first one, and a siren sounded the “all clear” signal. We returned to our factory building.
At the moment I sat down on my work stool, a powerful flash of light came through the small windows. We were blinded for about 30 seconds. Then, about 40 seconds after the flash, a loud sound and sudden change of air pressure followed. We were sure there was a huge explosion somewhere, but we didn’t know where. The sky was rapidly filling with dark clouds, and when I left the factory to walk home, about three miles away, a drizzling rain started. It was black rain. By the time I arrived home, my white shirt had turned gray. My grandmother quickly readied a bath for me. That bath might have saved me from the ill effects of the strong radiation that presumably existed in the black rain.
The next morning, a technical officer told us that the parachutes we had seen the day before contained measurement instruments and a transmitter. He also mentioned that there was serious damage in Nagasaki, but the details were unknown. The chief of the factory organized a rescue party. We tried to enter Nagasaki, but could not because the roads and the railroad were impassable. Later that afternoon, the railroad was opened to Michinoo, near Nagasaki station, and rescuers began to transport injured people to Isahaya and other cities.
On August 15, in a radio broadcast, Emperor Hirohito declared unconditional surrender. This was the first time that most Japanese citizens had heard the emperor’s voice. I think there was a widespread feeling of relief, and also fear for an uncertain future.
Many years passed before we had detailed information about the atomic bombs that were dropped on Hiroshima and Nagasaki. The Nagasaki bomb was a different type and far more powerful than the Hiroshima bomb. Even if the use of the Hiroshima bomb was justifiable in order to precipitate an end to the war, the bomb dropped on Nagasaki three days later was clearly a test of new arms. It cannot be justified.
The student mobilization ended, but I was not sure if my school record would allow me to enter a college. To receive some guidance, I visited my Isahaya school a week after the end of the war. Attached to the main gate were several sheets of large paper, on which numerous names were written. About half of the names were crossed off. Apparently the school was being used to accommodate the injured people of Nagasaki, who were listed on the papers. In the exercise field, several men were slowly strolling under the strong summer sun amid the noisy shrill of cicada. All were half-naked. When I looked closer, I saw that their skin was covered with something black with white specks. I thought it was a black medicine (I later found out it was dried blood). The white specks were maggots that had hatched on the human flesh. But that was not the most shocking sight. At the front of the gate, bodies covered with straw mats were stacked on a cart, probably to be taken to a crematory. Two people with a stretcher, a body on it, were coming from the school building. At that moment I noticed two half-naked people standing near the fence. They had been watching the loading of the bodies, a process that might happen to them in a few days. I felt as if I were seeing ghosts. My brain froze, the shrill of cicada faded, and my senses vanished. I think the mental shock I had at that sight, when I was 16 years old, had a certain permanent effect on me. I have regretted for a long time that I did not speak to these people; probably I didn’t have the courage at that time.
Nagasaki Pharmacy College
I tried to enter three different colleges in 1946 and 1947, but all rejected me. I didn’t have a strong school record to assist me, because I hadn’t studied even one day at the Isahaya school from which I graduated. I then heard that the Nagasaki Pharmacy College, a part of Nagasaki Medical College, was readying a temporary campus at a vacated military barrack near my home. (Both colleges had been completely destroyed by the atomic bomb.) I was admitted to the pharmacy college in April 1948. Although I was not planning to be a pharmacist, I didn’t have any other choice, under the circumstances. My grandmother presented me with a suit of silk clothes upon my entrance to college. There was no cloth to buy at the time, but she had a mulberry field. So she raised silkworms, reeled silk thread off the cocoons, dyed the thread, hand-wove the thread into cloth by herself, and then tailored the clothes.
Most of the pharmacy students lived in the dormitory, and they were always hungry due to an extreme shortage of food. I lived at home, and we owned farm land, so I was very lucky concerning food. Despite great effort by the professors, the resources and equipment at the pharmaceutical college were poor. We had a lot of difficulty carrying out experiments. Instruction in analytical chemistry and physical chemistry seemed fine, but I learned very little organic chemistry. In experiments on organic synthesis, we often set fire to solvents, due to poor technique and poor glassware. But many of us were experienced firefighters due to growing up in wartime, and we had no problem putting the fires out.
My interest in chemical experiments developed, but due to equipment limitations, the only experiments I could carry out were inorganic ionic reactions. With the permission of Professor Shungo Yasunaga (Fig. 2, left) of the Pharmaceutical Analysis Lab, I prepared many glass capillaries 1 mm in diameter, and packed them with alumina powder. When a small amount of a mixed solution of metallic ions was applied to the tip of the capillary and then it was dipped in a developing reagent, the reagent quickly rose up by capillary action. In the process, metallic ions were separated and colored bands appeared corresponding to the ions separated. It was a kind of chromatography. With Prof. Yasunaga’s permission, I brought back a small amount of various chemicals to my home and studied the conditions of separation in detail. The results were reported in the Journal of the Pharmaceutical Society of Japan a few years later, as my first paper (Yasunaga and Shimomura, 1953).
In March 1951, I graduated from Nagasaki Pharmacy School, at the top of my class. The school was reorganized as the Department of Pharmacy, Nagasaki University. Professor Yasunaga offered me a job there as an assistant in the analytical chemistry laboratory for students, and I took it. When I had spare time, I continued the chromatography experiments I had started when I was a student. I became interested in classical music after I heard an LP record somewhere. However, one LP cost nearly a month of my salary. Somehow, probably with my parents’ financial help, I managed to buy a record player and speakers, and I assembled an amplifier out of glass vacuum tubes, condensers and resistors.
Professor Yasunaga was a gentle and very kind person, trusted by people. After I had worked for him for four years, he obtained a leave of absence with pay for me, to study for one year. Moreover, he offered to introduce me to Professor Fujio Egami of Nagoya University, a well-known molecular biologist. We took the train to Nagoya, but unfortunately we found Professor Egami wasn’t at the university that day. Professor Yasunaga visited another person, Professor Yoshimasa Hirata (Fig. 2, center), an organic chemist. We chatted a few minutes. As we were leaving, Professor Hirata said to me, “Come to my lab. You may start at any time.” This was surprising, because we had just met. I didn’t know much about molecular biology or organic chemistry, so it didn’t matter to me which specialty I would study. I thought Professor Hirata’s words might be the direction given by heaven, and I decided to go to his lab. It seems that this decision determined my future, directing me to the studies of bioluminescence, aequorin and green fluorescent protein (GFP).
The Hirata lab and Cypridina luciferin
I enrolled in the Hirata laboratory, Department of Science, Nagoya University, as a research student in April, 1955. The Hirata lab was a wonderful place with a splendid atmosphere. Nobody taught me anything, but I learned much by watching other people and by independent study.
On my first day, Professor Hirata brought out a large vacuum desiccator and said, “This contains dried Cypridina.” He explained to me that Cypridina, a small crustacean common in shallow coastal waters of Japan, emits light with an organic compound called luciferin and an enzyme, luciferase. He told me that luciferin is extremely unstable and rapidly decomposes in the presence of oxygen, and also that Professor Newton Harvey of Princeton University had been trying to purify luciferin for the past 20 years but had been unsuccessful. Professor Hirata asked, “Could you purify and crystallize Cypridina luciferin for the purpose of structure determination?” At the time, crystallization was the only practical way to prove the purity of a substance. Professor Hirata also told me that he could not give this project to a student pursuing a degree, because the outcome was so uncertain. I clearly understood the difficulty of the work. Since I was there for study, not for a degree, I replied, “I would like to do my best.”
This did, indeed, turn out to be a very difficult crystallization. It took me ten months of extremely hard work to extract, purify, and crystallize the luciferin. When I finally succeeded, I was so happy I couldn’t sleep for three days. Since the end of the war, my life had been dark, but this gave me hope for my future. Probably the greatest reward I gained was self-confidence; I learned that any difficult problem can be solved by great effort. With the crystallization accomplished, my stay in Nagoya was extended one year to study the structure of Cypridina luciferin (Fig. 3). Our first paper on Cypridina luciferin was published in 1957, although the chromophore structure of luciferin remained to be elucidated.

To America
In the spring of 1959, I received a letter from Dr. Frank Johnson (Fig. 2, right) of Princeton University inviting me to work at his laboratory. When Professor Hirata heard about my plan to go to Princeton, he awarded me a doctoral degree for my Cypridina work, even though I wasn’t enrolled as a doctoral student. Prof. Hirata knew having a doctorate would double my salary at Princeton. I was completely surprised, and I accepted his offer with thanks. I applied for and received a Fulbright travel grant, which provided me with various experiences unavailable otherwise.
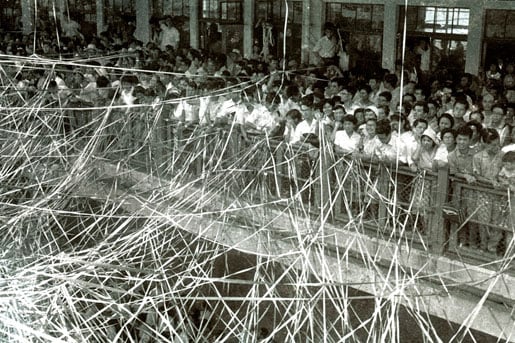
On August 4, 1960, Akemi Okubo and I got married in a traditional way, arranged by a match-maker. Akemi was a graduate of the Pharmacy Department where I worked. On August 27, I left Yokohama on a ship bound for Seattle, along with more than 200 other Fulbright fellows and students, but Akemi could not accompany me due to a visa problem. The day was my 32nd birthday. Since that voyage was the last Pacific cruise of the ship Hikawa-maru, the pier was filled with people (Fig. 4). Thousands of colored tapes connected people on the boat with well-wishers on the pier. I will never forget the scene when the ship started to move, and the tapes broke and then fell. After traveling by rail across the United States, I arrived at Princeton on September 17, 1960, and stayed at Dr. Johnson’s house that night. The next morning, Dr. Johnson offered to help me find an apartment. We saw a newspaper advertisement for a room for rent, and we stopped at the house. A man responded to the doorbell, but he quickly shut the door when he saw my face. It was a clear case of racial discrimination that I rarely encountered.
On my first visit to Dr. Johnson’s office, he took out a small vial containing white powder, and said “This is the freeze-dried light organs of the luminous jellyfish Aequorea, and it should emit light when mixed with water.” We went into a dark room and tested it, but we could not see any light. However, he enthusiastically explained to me that Aequorea were very abundant in Friday Harbor, Washington State, and that they were brilliantly luminous. Then he asked if I would like to study the bioluminescence of this jellyfish. I answered, “I will be glad to do it.” Thus, we decided to go to Friday Harbor the following summer.
During my first few months in Princeton, I extracted and purified luciferase from dried Cypridina. I was surprised at the large quantity of dried Cypridina that was stocked in the Princeton biology department, which included sealed bottles dated 1928 and a sealed tin can bearing the name of Sakyo Kanda, a pioneer in the study of bioluminescence. There was also a large amount that had been collected for intended military use by the Japanese army during the war, seized by the U.S. Navy at the end of war and then donated to Professor Harvey’s lab. Dried Cypridina, even if it is many decades old, luminesces when crushed and mixed with water.
Miss Yo Saiga arrived in December 1960 to work as our research assistant, and my wife, Akemi, arrived the next month just after the inauguration of President John F. Kennedy, after a delay of four months due to her visa problem.
On June 23, 1961, Dr. Johnson, my wife, Miss Saiga and I departed for Friday Harbor. Dr. Johnson had purchased a new Plymouth station wagon for the trip. We loaded it with a large photometer, other instruments and chemicals needed for research, and suitcases for four people on the roof. It took us seven days to cross the continent, with Dr. Johnson driving the whole way. Upon arrival, we met our host, Dr. Robert Fernald, at the Friday Harbor Laboratories, University of Washington.
Friday Harbor is on San Juan Island, which lies east of the city of Victoria on Vancouver Island. The scenery of San Juan Island was splendid. The sea was clean and beautiful. At low tide, there were colorful sea urchins and starfish in various sizes scattered on rocks, and some abalones. There were also abundant fish. We could easily get rock fish of about 30 cm long using simple lures from a boat or rocky shore. We often ate them as sashimi. Indeed, Friday Harbor at the time was a kind of paradise for us.
For research space, Dr. Fernald assigned us part of a large laboratory. There were three other scientists in the room, and one of them was Dr. Dixy Lee Ray, a professor at the University of Washington at the time, and later chairperson of the U.S. Atomic Energy Commission and governor of Washington State. She was always accompanied by her dog, even though dogs were prohibited in Friday Harbor Laboratory by a state law. She declared that the animal was her assistant.
Our research material, Aequorea, was really abundant. A constant stream of floating jellyfish passed along the side of the lab dock every morning and evening, riding with the tidal current. I describe our methods for collecting and extracting the luminescent material from Aequorea in my Nobel lecture.
We brought the crude extract back to Princeton in September 1961, where we began the process to purify it. In February 1962, we obtained about 5 mg of nearly pure luminescent substance. It was a protein, and we named it aequorin. Aequorin attracted wide attention as a unique protein that emits light in the presence of calcium ions, even in the absence of oxygen. Aequorin was the first photoprotein ever discovered. During the column chromatography of aequorin, we found a trace of protein that showed green fluorescence, which eluted sooner than aequorin. We also purified that protein, which is now called green fluorescent protein (GFP).
In August 1962, I visited Bermuda to study the famous fireworm Odontosyllis enopla at the Bermuda Biological Station. The fireworms are very small but they show a spectacular bioluminescence display that is correlated with the lunar cycle. The luminescence display takes place during a period of several days following a full moon. It begins about one hour after sunset and lasts only for 10 minutes. It begins with the sudden appearance of a swarm of brilliantly luminescent females (about 2 cm long) at the surface of the water, each worm quickly moving in a tight circle. Within a few seconds, numerous brightly luminescent males (about 1 cm long) appear, and they dart toward the females from all directions, attracted to the light. I collected the fire-worm and studied the properties of its luciferin.
Due to my U.S. visa expiring in 1963, I was expected to return to my position in the Pharmacy Department at Nagasaki University, from which I was on leave. However, I received through Professor Hirata an offer of the position of associate professor at the Water Science Institute, Nagoya University, with the understanding that I could continue my research on bioluminescence. I took the offer. In September 1963, I began working under Professor Tadashiro Koyama at the Water Science Institute. A few months later, Professor Hirata asked me to assist his graduate student, Yoshito Kishi (later a professor at Harvard University) to determine the structure of Cypridina luciferin. In the summer of 1964, Kishi and I went to Setoda in the Inland Sea to do mass collection of Cypridina, taking some 10 students with us. From frozen Cypridina, we extracted luciferin and then purified and crystallized the luciferin. With that material, Kishi successfully determined the structure of the luciferin the next year.
In February 1965, I went to New Zealand to study two kinds of bioluminescent organisms: the cave worm Arachnocampa and the freshwater limpet Latia. Soon afterwards, I decided to go back to Dr. Johnson’s lab at Princeton University, because I realized I did not have sufficient ability to do top-level work in two unrelated fields, earth science and bioluminescence. I wanted to study and clarify the chemical mechanism of aequorin bioluminescence, since some people doubted the existence of a photoprotein like aequorin. In December 1965, I returned to Princeton with my wife and our one-year-old son, Tsutomu.
Since the early 1950s, it had been believed that calcium ions play important roles in living bodies, but there had been no way to demonstrate this experimentally. In 1967, however, Ellis Ridgway and Christopher Ashley, University of Oregon, experimentally proved the involvement of calcium ions in the contraction of muscles using aequorin as an indicator. As the usefulness of aequorin as a calcium probe increased in the fields of biology and physiology, I wanted to clarify the mechanism of aequorin luminescence to assist in its use.
I already knew that the aequorin luminescence was caused by an intramolecular reaction that takes place in the protein molecule, which would be difficult to study. But I decided to do my best. It turned out to be an unexpectedly large undertaking. It took about 12 years to obtain a structural model of aequorin, as detailed in my Nobel lecture. We needed a large amount of aequorin to carry out this study, so we returned to Friday Harbor every summer for more than 10 years, where we collected and processed about 3,000 jellyfish a day. My staff, my wife, my children, and some local students that we hired were of great assistance in the collection and processing of Aequorea. Dr. Johnson devised a “jellyfish-cutting machine” to speed up the process of cutting the ring from the animals. In 1972, back at Princeton, we succeeded in determining the structure of AF350, a part of the aequorin chromophore. By 1978, we had achieved a general understanding of the aequorin luminescence reaction.
Between 1965 and 1978, in addition to my work with aequorin, I also did research on the bioluminescence of various luminous organisms including the limpet Latia; the krill Meganyctiphanes; the worm Chaetopterus; the firefly squid Watasenia, various coelenterates, and luminous bacteria. I also studied the properties of GFP and resolved a controversy regarding the role of a dioxetane intermediate in the luminescence reaction of firefly luciferin. Those matters are detailed in my book (Shimomura, 2006).
To the Marine Biological Laboratory, Woods Hole
Dr. Johnson retired from Princeton in 1977, and I decided to move to a marine laboratory. Before leaving Princeton, I elucidated the chromophore of GFP (Shimomura, 1979). I also performed research on dinoflagellate luciferin and luminous scale worms. In 1981, with the kind arrangement made by Dr. Woodland Hastings of Harvard University and Dr. Benjamin Kaminer of Boston University, I moved to the Marine Biological Laboratory (MBL) in Woods Hole, Massachusetts, where I was a Senior Scientist. My wife, Akemi, worked at the MBL as my research assistant. I also accepted an adjunct professorship at Boston University Medical School.
Established in 1888, the MBL is the oldest marine laboratory in the western hemisphere. Over the years, many Japanese scientists have performed research at the MBL, including historic names such as Shosaburo Watase, Umeko Tsuda, Hideyo Noguchi, Sakyo Kanda, Katsuma Dan, Sachio Hiramoto, and Shinya Inoué. At the MBL, I continued my work to extract, purify and study the luminescent substance of many organisms, including the millipede Luminodesmus; the brittle star Ophiopsila; and several types of luminous mushrooms.
Since 1975, aequorin had become widely used among cell biologists and physiologists as an excellent calcium probe, and its applications peaked around 1985. Since I was practically the only source of aequorin in this time period, I sent out several hundred aequorin samples in response to requests from investigators all over the world. The cDNA of aequorin was cloned and recombinant aequorin was made in 1985, but the patent owners of recombinant aequorin, the University of Georgia and Chisso Corporation of Japan, were not sure about expanding the use of aequorin, thus the general use of recombinant aequorin was delayed. In 1988, in collaboration with Professor Yoshito Kishi of Harvard, we succeeded in making various aequorins that had different calcium sensitivities and properties. Those aequorins contained various derivatives of coelenterazin instead of coelenterazine in their molecules, and were called semi-synthetic aequorins. In 1995, I undertook work on the X-ray structure of aequorin, and the three-dimensional structure of aequorin was obtained in 2000 (Head et al., 2000).
In 1992, the cDNA of GFP was cloned by Dr. Douglas Prasher, who was then at Woods Hole Oceanographic Institution. At that time, however, it was commonly believed that expressing the cDNA in living organisms would not produce fluorescent GFP, because the formation of its chromophore requires the reactions of condensation and dehydrogenation that are not expected to occur spontaneously. In 1994, however, Dr. Martin Chalfie of Columbia University tried to express the cDNA in E. coli and a nematode worm, and he and his colleagues unexpectedly observed the fluorescence of the expressed GFP. The results suggested GFP, and other proteins linked to GFP, could be expressed in living organisms to observe their behavior. Dr. Chalfie’s work attracted the interest of many people, triggering rapid progress in the applications of GFP. Dr. Roger Tsien of University of California, San Diego, engineered GFP by modifying the amino acid residues surrounding the chromophore, producing many different fluorescent proteins that emit various colors, from blue to red. Today, GFP is widely used as a fluorescent marker of protein molecules and cells, and it is an essential tool in the study of biology, physiology, and medicine. GFP has been used also in other fields, such as the detection of cadmium, zinc, and explosive TNT and fumes (Zimmer, 2005). The range of applications of the fluorescent proteins is beyond imagination.
After my retirement
I retired from the Marine Biological Laboratory in 2001. Because I wanted to do some more experiments and also I was still supplying aequorin samples to people upon request, I moved all my laboratory equipment and chemicals to my home, where I set up my Photoprotein Laboratory. My retirement symposium, “GFP and Aequorin,” was held at the MBL’s Lillie Auditorium on July 27, 2002, through the kind arrangement of Dr. Shinya Inoué, a world authority on microscopy. Among the attendees were Martin Chalfie and Roger Tsien. I started to write a book for the next generation students who want to explore the chemistry of bioluminescence. The book Bioluminescence: Chemical Principles and Methods was published by World Scientific Press in 2006. The book contains a comprehensive overview and chemical information on all known bioluminescence systems, comprising 35 different types of bioluminescent organisms.
The summer of 2004 was splendid. In July, I received the Pearse Prize from the Royal Microscopical Society for the discovery of GFP. In early August, I gave an invited lecture at the International Bioluminescence and Chemiluminescence Symposium. In the end of August, I attended the symposium, “Calcium-Regulated Photoproteins and Green Fluorescent Protein”, which was held in my honor at the Friday Harbor Laboratories as part of the lab’s centennial celebration. At this symposium, almost all the well-known researchers in bioluminescence and related fields gathered from all over the world, including Martin Chalfie, Roger Tsien, Shimya Inoué and Atsushi Miyawaki. I was happy to see many of my old friends, though I was a little sad to observe that the sea at Friday Harbor was steadily being polluted. In 2006, I received the Asahi prize, one of the most prestigious prizes in Japan.
I would like to add a note on the jellyfish Aequorea at Friday Harbor. Between 1961 and 1988, we traveled to Friday Harbor and back to the East Coast 19 times (13 of which were road trips) and collected a total of about 850,000 Aequorea specimens to obtain aequorin for my research. The jellyfish were always abundant during that period. Mysteriously, however, they suddenly decreased in number after 1990, and it became very difficult to collect even a few. The cause of this drastic decrease could be pollution of the sea bed by crude oil spilled from the tanker Exxon Valdez in 1989, or it could be a natural cause. If the disappearance of the jellyfish had occurred 20 years earlier, we wouldn’t have been able to learn the mechanism of the aequorin bioluminescence reaction, as well as the chromophore of GFP.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Osamu Shimomura died on 19 October 2018.Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
