Richard F. Heck
Biographical
Richard F. Heck: Nobel Laureate in Chemistry
 by Luke R. Odell and Mats Larhed
by Luke R. Odell and Mats Larhed
Richard F. Heck was born in Springfield, Massachusetts, U.S.A. on August 15, 1931. The only son of a housewife and a salesman, he moved to Los Angeles, California at the age of eight. His passion for chemistry began in his early teens and stemmed naturally from his interest in growing orchids. His interest developed throughout high school and culminated in his majoring in Chemistry at the University of California, Los Angeles (UCLA). He received his bachelor’s degree in 1952 and immediately commenced his graduate studies under the supervision of Professor Saul Winstein. Heck was drawn to the complexity and versatility of the area and particularly enjoyed the “way you can make all sorts of compounds”.1 He received his Ph.D. in 1954 in physical organic chemistry, and his main research area was neighboring group participation in the solvolysis of arylsulfonates. A National Science Foundation Postdoctoral Fellowship took him to the Swiss Federal Institute of Technology in Zurich, where he worked with Professor Vladimir Prelog, who became a 1975 Nobel Laureate. During his one year stay he carried out research on the solvolysis of medium sized cycloalkyl arylsulfonates. In 1955, Heck returned to UCLA and continued his research on neighboring group effects, an area which is now included in all organic chemistry textbooks.
In 1956, Heck went to work for the Hercules Powder Co. (now Ashland Inc.) at their research center in Wilmington, Delaware. His strong physical organic chemistry background undoubtedly influenced their decision to hire the then 25 year old. His first project involved working on the development of a commercial process for producing polymers using Ziegler-Natta catalysts (this process is still used today to produce large volumes of rubbers and plastics). Although Heck accomplished “little of scientific value in the two years that I was in this program”2 the experience he gained in transition metal chemistry would prove to be invaluable in his next project. In a defining moment, his supervisor, Dr. David Breslow, suggested that Heck “do some thing with transition metals”. Few could have foreseen that this suggestion would transform modern organic chemistry and give rise to the vast and important field of transition metal catalysis.

Figure 1. Richard F. Heck in the laboratory.
In 1958 organotransition metal chemistry was still in its infancy. There were several important transition metal-catalyzed processes used commercially, but very little was known about the actual chemistry involved. Heck’s initial idea was to investigate the chemistry in one of these processes and to use this knowledge in the development of new chemical transformations. This work began with an investigation of the hydroformylation process, which ultimately resulted in a mechanistic rationalization of this reaction. Today Heck’s work on the hydroformylation reaction is widely regarded as the first correct transition metal-catalyzed reaction mechanism. This was a significant achievement and provides an excellent insight into the way Heck approached his research. At a time when most people saw transition metal catalysis as a ‘black box’ (an unknown method that could be used to aid certain chemical processes), Heck concerned himself with finding out what was inside the box (i.e. how these reactions worked) and using this information to discover new chemistry.
Despite the discovery of many new chemical reactions based on his newfound mechanistic understanding, Heck had no idea how Hercules could profit from these discoveries. This forced Heck to take his research in a new direction and, once again, it was a discussion with a colleague that would dictate Heck’s next move.
Pat Henry, who worked in the laboratory opposite Heck, was studying the mechanism of the industrially important Wacker Process. Heck was intrigued by Henry’s notion that decomposition of the intermediate palladium species occurred via a β-hydrogen elimination. Heck then proceeded to “see what would happen” if an organopalladium species, which lacked a β-hydrogen, was prepared in the presence of another molecule. His very first experiment was an overwhelming success (Figure 2). In a description of this seminal study Heck stated that he added
phenylmercuric acetate to a stirred solution of tetrachloropalladate … under an atmosphere of ethylene. An immediate reaction occurred. Palladium metal precipitated and ethylene gas was rapidly absorbed. Analysis of the reaction mixture showed that about an 80% yield of the styrene and 10 % yield of trans-stilbene had been formed.2
This was an incredible finding and marked the discovery of a new carbon-carbon bond forming reaction, which we today know as the Heck reaction. Heck then proceeded to systematically investigate the unique reactivity of these organopalladium compounds with carbon monoxide, alkenes and dienes. This work was documented in a remarkable series of seven consecutive articles in the highly regarded Journal of the American Chemical Society. Of great synthetic importance was the compatibility of these processes with almost all common organic functional groups. However, the reaction had a number of significant drawbacks. It required the use of highly toxic mercury or tin salts and stoichiometric amounts of expensive palladium, two factors which significantly limited the usefulness of this reaction. These issues became the focus of Heck’s next research phase.
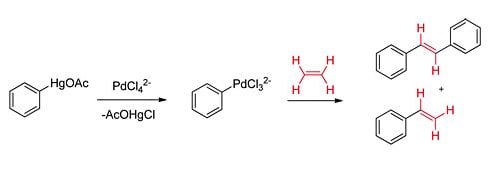
Figure 2. Heck’s seminal olefin arylation experiment.2
In 1971, Heck left Hercules and accepted a faculty position at the University of Delaware. Over the next few years Heck worked towards developing conditions to make the reaction more ‘user friendly’. Inspired by reports on the successful formation of the haloaryl palladium-phosphine complexes, Heck explored whether these intermediates could replace the problematic stoichiometric arylmercury-palladium combination. He also investigated the effects of certain bases on the reaction, which led to the development of a reaction that was catalytic in the amount of palladium required. This discovery, reported in 1972, formed the basis for a number of systematic studies on the application of this reaction in organic synthesis. Today, this reaction is known by chemists all over the world as the Heck reaction (or Heck-Mizoroki reaction). In addition to the discovery of the Heck reaction, Heck and his team also invented three other very useful palladium-catalyzed carbon-carbon bond forming reactions over the next few years. The palladium-catalyzed arylation of alkynes was reported by Heck in 1975 and later that year Sonogashira reported that the addition of copper salts resulted in a faster reaction. This reaction is known today as the Sonogashira reaction and is one of the premier methods for functionalizing alkynes. Heck also developed a novel carbonylative methodology for the preparation of aryl carboxylic acid derivatives from an aryl halide, a nucleophile and carbon monoxide. Finally, Heck reported the coupling of vinylboronic acid with acrylates. This can be seen as a precursor of the extremely important Suzuki coupling and the oxidative Heck reaction.
The importance of the Heck reaction grew slowly among organic and medicinal chemists. The current impact of Heck’s discoveries can be clearly seen by performing a full text search in a chemical database. A search of Heck’s name in SciFinder gives 1,298 hits for the years 2000−2005 and 3,845 for 2005–2011 (as of April 27, 2011). The continued development and use of the Heck reaction has now been summarized in more than 40 reviews. The first special issue of a scientific journal focusing only on the Heck reaction was published in 2006 and the first book on the Heck reaction was published in 2009. Today, the Heck reaction is an important concept and tool for all organic chemists and medicinal chemists. Organopalladium chemistry has achieved a position of prime importance through its operational simplicity, enormous compatibility with sensitive functional groups and broad applicability, from materials science to the synthesis of drug candidates and approved drugs. Almost all sub-disciplines of modern organic chemistry have benefited from advances in the field. Heck’s work can be regarded as a precursor to a number of other Pd-catalyzed cross-couplings, including those with boronic acids (known as the Suzuki cross-coupling), organotin (known as the Stille cross-coupling), organonickel compounds (known as the Kumada-Corriu cross-coupling), organozinc compounds (known as the Negishi cross-coupling) and organosilicon compounds (known as the Hiyama cross-coupling), and linkages with alcohols and amines. Undergraduate students learn about the Heck reaction and perform experiments based on it in the laboratory. Process chemists perform palladium-catalyzed reactions in the large scale manufacture of fine chemicals, fragrances, pesticides and pharmaceuticals. Of all the chemistry developed by Heck, maybe the greatest social impact has been from the Pd-mediated coupling between an alkyne with an aryl halide. This is a reaction that is used for fluorescence labeling of DNA bases, which has contributed to the automation of DNA sequencing and sequencing of the genome. Heck’s work has motivated thousands of researchers to explore the unique possibilities of palladium catalysis in their own work. Richard Heck laid the foundation for virtually all the metal-catalyzed coupling reactions that are an essential component of modern organic synthesis.

Figure 3. Richard F. Heck was awarded an honorary doctorate from Uppsala University in 2010. Richard F. Heck in first row. Second row from left: Professor Walter Cabri, Professor Anders Hallberg (President of Uppsala University), and Professor Mats Larhed. All three devoted palladium chemists.
After an extraordinary and prolific career, Willis F. Harrington Professor Richard F. Heck retired from the University of Delaware in 1989, where he remains a Professor Emeritus. In 2005, he became the recipient of the Wallace Carothers Award, which recognizes creative applications of chemistry that have had substantial commercial impact. In 2006, he received the Herbert C. Brown Award for creative research in synthetic methods from the American Chemical Society. That same year he also returned to the laboratory as a visiting professor at Queen’s University, Canada. In 2010, he received an honorary doctorate from Uppsala University, Sweden. Richard Heck has published over 200 scientific papers and has an H index of over 55 (counting from 1987, he retired in 1989).
Richard now lives in the Philippines with his wife, Socorro, whom he met at a Manila restaurant while visiting the Philippines in 1979. Richard’s life has now come full circle and just as he did as a teenager in Los Angeles, he spends his spare time growing orchids.
References
1. Masunaga, S. Alumnus Richard Heck wins Nobel Prize in Chemistry, UCLA Daily Bruin, October 13, 2010 (accessed April 2011).
2. Heck, R. F., “Cobalt and Palladium Reagents in Organic Synthesis: The Beginning,” Synlett 2006, 2855.
The text is based, in part, on discussions with Professor Richard F. Heck, on the concise biography by Nobel Laureate Ei-ichi Negishi (J. Organomet. Chem 1999, 576, xv), and on the editorial by Professor Douglass Taber and Professor Victor Snieckus (Synlett 2006, issue 18).
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Richard F. Heck died on 9 October 2015.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
