by David M. States*
Ludolf von Krehl’s search for colleagues who would integrate the then quite separate disciplines of the natural sciences under the umbrella of biomedical research led him to Otto Meyerhof. Meyerhof’s study of intermediate metabolism involved a mix of physiology, pharmacology, physics and pathology. His already well-known successes with the study of muscle physiological chemistry, for which he had won a Nobel Prize in 1922, made him a logical choice as head of the KWImF’s proposed Physiology Institute.

Otto Meyerhof
Photo: Courtesy of Walter Meyerhof, son of Otto Meyerhof
The years Meyerhof spent at the KWImF represent the most important and productive phase of his brilliant career. Indeed, some scientific historians, including Thomas Kuhn, believe the work during the 1930s in the laboratories of Meyerhof, Parnas, Embden, Warburg and a few others was the mark of true scientific revolution. While in Heidelberg, Meyerhof and his assistants published over 200 articles and played a central role in piecing together the complex puzzle of glycolysis – a major milestone in the study of intermediary metabolism. Meyerhof and his colleagues not only discovered a major proportion of the chemical compounds involved in this metabolic pathway, but played a key role in determining how and in which distinct sequence those compounds chemically interact. In the process, they pioneered our understanding of how energy is biochemically transformed, stored and released for work in the cell.
Meyerhof attracted a steady stream of remarkable assistants and students to the KWImF in Heidelberg. The chemist Kurt Lohmann was clearly Meyerhof’s right hand man during this time. It was he who first discovered ATP – the molecule now known as the universal energy donor. Lohmann and Meyerhof also made the critical structural and functional studies of this all-important molecule while at the KWImF. The Physiology Institute was, however, far more than a two-man show. Hermann Blascko, David Nachmansohn, W. Kiessling, Paul Ohlmeyer, Severo Ochoa, Fritz Lipmann, George Wald and André Lwoff all worked in Meyerhof’s laboratory during the 1930s. The latter four went on to win Nobel Prizes of their own after leaving the KWImF. These men and other students of Meyerhof enthusiastically took forward the torch of the Meyerhof School to other institutes and nations in the years that followed.
Prelude to the study of intermediate metabolism
The forerunners to Meyerhof’s work on intermediate metabolism can be traced back to the late 18th century, when Lavoisier introduced the concept of respiration as the combustion of carbon and hydrogen in the lungs. By the mid-19th century, scientists, especially in France and Germany, had formulated critical questions about how organisms gradually break down and use foodstuffs. Yeast and muscle were the focus of most of these early studies. Glycogen, the sugar that figures so prominently in the metabolic pathway linked with Meyerhof’s name, had been discovered in the 1850s and oxidation and fermentation were proposed as the mechanisms by which such sugars are utilized. Lactic acid, the focus of many of Meyerhof’s early studies – was known to accumulate in muscle tissue and was considered a by-product of fermentation in muscle, much as alcohol and carbon dioxide are by-products of yeast fermentation.
Progress slowed during the second of half of the 19th century, although critical advances in inorganic and organic chemistry helped to usher in a new era for physiological chemistry. Just before the turn of the century, scientists began to forcefully formulate the chemical reactions that occur within cells. In 1897, Eduard Buchner provided a major turning point with celebrated studies on alcoholic fermentation. Buchner had isolated the enzyme responsible for fermentation from yeast-press juice. This enabled him to do controlled experiments with cell-free chemical fermentation. This was the first demonstration of biological processes outside of the living cell. Buchner’s work was important for several reasons: first, it discounted long held and popular vitalistic theories that considered cellular processes as fundamentally different from other principles of chemistry; second, it introduced a methodology that would allow scientists to break down biochemical processes into their individual steps; and, finally, the discovery of cell-free fermentation had opened the doors to one of the most important concepts in biochemistry – the enzymatic theory of metabolism.
In 1906, Harden and Young elaborated upon Buchner’s work by showing that complementary enzymes – coenzymes – are also crucial to such processes. During the next decade, scientists like Carl Neuberg, Gustav Embden and Jacob Parnas made major contributions to early metabolic studies in yeast. The answers to how such processes work would take another three decades, but methodology and the way scientists formulated their questions about metabolism had entered an entirely new phase.
The beginning of Meyerhof’s career in science
Ludolf von Krehl was building up a small research program on metabolism at his University of Heidelberg Medical Clinic at the same time that Otto Meyerhof was finishing up his medical studies in Heidelberg. Otto Warburg, who had been a student of Emil Fischer, had joined Krehl in 1906. When Krehl offered Meyerhof his first research position in 1909, it was Warburg’s responsibility to teach the newly graduated physician his techniques for investigations of respiration, oxygen consumption and growth rates in sea urchin eggs. Warburg’s innovative ideas and dynamic, self-confident approach had a dramatic impact on Meyerhof, inspiring him to focus his career on physiological chemistry. Meyerhof worked at Krehl’s laboratory for little over two years, but the establishment of ties with Krehl and development of a close friendship with Warburg were to be factors which would continue to shape Meyerhof’s career.
After leaving Heidelberg, Meyerhof took a position at the University of Kiel, where he quickly began to make a name for himself. In 1913, he presented an epoch making lecture on the energetics of living cells. This was one of the very first adaptations of the physical laws of thermodynamics to physiological chemistry. Meyerhof’s goal was to understand how energy is transformed during chemical interactions in the cell. He recognized that between initial energy input via food and its final dissipation as heat, a series of intermediate steps to transform that energy must occur to maintain the organism in a state of dynamic equilibrium. With minor revisions, his theory on the thermodynamics of living matter remained influential for decades.
In his ensuing efforts to relate energy transformations and chemical changes to cellular function, Meyerhof turned his attention increasingly toward experimentation with muscle, where such transformations promised to be large enough in scale to test his new theory.
Meyerhof was also interested in analogies between oxygen respiration in muscle and alcoholic fermentation in yeast and the role that enzymes played in both. 1918 marked the first experimental milestone in Meyerhof’s career, when he showed that a coenzyme involved in the production of lactic acid in muscle was the same coenzyme as Harden and Young had found involved in alcohol fermentation in yeast. This was important early evidence of the unity in life of fundamental biological processes.
In his 1913 address, Meyerhof had mentioned the work of the Englishman, A.V. Hill. Hill had pioneered methods to measure heat production in biological processes. Since Meyerhof’s lecture, Hill had found a pattern of discrete temperature changes during muscle contraction and relaxation that suggested a complicated series of biochemical interactions. This reminded him of work by Fletcher and Hopkins in 1907, which had shown that lactic acid increases in resting muscle in an oxygen-free environment, but then disappears when oxygen is reintroduced. Hill noted that his own measurements of heat during anaerobic conditions correlated strikingly to Fletcher’s and Hopkins’ results. This was important evidence for the theory that lactic acid was not simply a by-product of muscle activity, but must be a part of the muscle machinery itself.
Soon after the end of WWI, Meyerhof began collaborating with A.V. Hill. Both men were convinced that a key to understanding metabolism lies in quantitatively correlating data on heat development, mechanical work and cellular chemical reactions. In Germany, Meyerhof focused on chemical methods to measure oxygen consumption, the conversion of carbohydrates, lactic acid formation and decomposition, then correlating it to thermodynamic data and various phases of muscle activity.
Between 1918 and 1922, Meyerhof worked out an extraordinary amount of this biochemical detail, including proofs that it is glycogen that is converted into lactic acid in the absence of oxygen. He also showed that in the presence of oxygen, only one-fifth to one-fourth of lactic acid production during anaerobic contraction of the muscle is subsequently oxidized to carbon dioxide and water. Thus, Meyerhof tied the release of energy during this particular oxidation to the reconversion of the remaining four fifths of the lactic acid back to glycogen.
These results had several important ramifications: they explained the course of heat production measured by Hill; and they confirmed and extended a famous theory of Pasteur’s that less glycogen is consumed in muscle metabolism in the presence of oxygen than in its absence. The depression of glycolysis by respiration was thereafter referred to as the Pasteur-Meyerhof effect. This would be significant later on in working out the full details of the glycolytic pathway. Finally and most importantly, the conversion of glycogen to lactic acid and back again to glycogen was the first evidence of the cyclical character of energy transformations in living cells. Meyerhof called it the lactic acid cycle. Meyerhof and Hill’s analysis of this cycle and its relation to respiration earned both men the Nobel Prize in 1922.
A Nobel Prize winner struggles for respect: Warburg and the KWG come to the rescue
Meyerhof’s early achievements were amazing given the conditions under which they were performed. WWI interrupted his experimentation and delayed contact with Hill. He worked virtually alone during his years in Kiel; the one exception was a six-month period after his Nobel Prize was announced, during which time Hans Hermann Weber served as his assistant (this fact is notable because Weber was later called upon to direct the MPImF Physiology Institute in the 1950s). Anti-Semitism and resentment over Meyerhof’s pacifism during the First World War were largely responsible for the conspicuous lack of support from the faculty at Kiel. Despite his Nobel Prize and solid recommendations from outside referees and his department chair, Meyerhof was denied promotion at the University. Indeed, he encountered great difficulty finding a suitable position anywhere in Germany during this period.
Meyerhof became depressed and was at the point of emigration when his old friend Otto Warburg, who now directed one of the Kaiser Wilhelm Institutes in Berlin-Dahlem, began lobbying KWG President Adolf von Harnack on his behalf. In late 1924, Warburg made Meyerhof a stop-gap offer of space in his own institute.
Although the facilities Warburg offered were cramped, they were a major improvement over the instability of Kiel. And with its collection of brilliant physicists, chemists and biologists, the complex of KWIs in Berlin-Dahlem promised an intellectually stimulating environment for Meyerhof. He accepted and during the next five years was able to build a small research team to help him unravel the lactic acid cycle. Thus, it was in Berlin that Meyerhof began to truly lay the groundwork for the brilliant successes that were to follow at the KWImF in Heidelberg during the 1930s.
Two noteworthy scientific trends that were taking place during this time should be mentioned. The first revolved around the recognition of the importance of enzymes in the metabolic pathways and corresponding methodological advances in enzyme chemistry. Many research groups throughout the world made significant contributions in this regard, but Berlin-Dahlem was a particular center for this work, with the Big Three of Warburg, Neuberg and Meyerhof working in close concert with one another. The second trend began with the discovery of a compound called creatine phosphate. This excited new interest in the possible role that phosphates might play in energy transfers and set many other research groups in a search of similar phosphorylated compounds that might be involved in yeast fermentation and muscle glycolysis.
With the introduction of thermodynamics, advances in enzyme chemistry and the discovery of phosphorylated compounds in the 1920s, the stage was set for a sudden shift in how scientists understood intermediate metabolism. That shift would take place almost immediately after the arrival of Meyerhof at the new KWImF.
Meyerhof is reunited with Krehl in Heidelberg
Otto Warburg had been Ludolf von Krehl’s first choice as director of the KWImF Physiology Institute. And although Warburg decided to stay in Berlin, he remained a close advisor to Krehl and the KWImF throughout the late 20s and 30s. Given Meyerhof’s subject area, his credentials and the inadequacy of facilities in Berlin-Dahlem befitting his international stature, it is not surprising that Warburg strongly encouraged Krehl to offer the position to his friend and trusted colleague. It helped, of course, that Krehl knew Meyerhof personally from his earlier years in Heidelberg, but accepting a Nobel Prize winner as second choice was hardly a bitter pill for Krehl to swallow. The avalanche of results in Meyerhof’s institute during the next 8 years would certainly prove the worthiness of the choice.
For his part, Meyerhof never hesitated at Krehl’s enticing offer. Given the exciting intellectual environment that existed at Berlin-Dahlem, Meyerhof’s excitement was a testament both of his respect for Krehl’s vision and the magnificence of the new facilities. The reunification with Krehl seemed to be a dream come true. Finally, after so many years of struggling against non-scientific barriers, Meyerhof believed he would be able to concentrate his full attention upon his work.
Meyerhof’s assistants were crucial to his success in Heidelberg. Fortunately, he was able to bring with him the critical members of his team from Berlin-Dahlem. This minimized the transition time. As it turned out, those first months were extremely important. The original group included Kurt Lohmann, the expert chemist who worked with Meyerhof for nearly 13 years, Fritz Lipmann, David Nachmansohn, Hermann Blaschko, and Severo Ochoa, as well as his trusted technician, Walter Schulz. Meyerhof’s reputation for innovation and his holistic approach also served as a magnet for other talented young scientists who made major independent contributions at the KWImF. During the next eight years, Ken Iwasaki, Paul Rothschild. M. Dubuisson, George Wald, Alexander von Muralt, André Lwoff, W. Kiessling, H. Lehmann and Paul Ohlmeyer took their turns at the Physiology Institute. Frequent visits by guest scientists, such as Einar Lundsgaard, A.V. Hill, Otto Warburg, and Hans Krebs also contributed greatly to the intellectual mix.
Meyerhof’s broad philosophical approach to his work and personal generosity set the tone for an enthusiastic collaborative environment that evolved at his institute. His research group had a tremendously international flavor. Discussions were frequent, open and unusually informal for the time. This included, especially for younger scientists at the laboratory, frequent contacts with colleagues in Richard Kuhn‘s chemistry institute. Years later, many of the scientists who had worked with Meyerhof during these years fondly recalled their time at the KWImF, both as a tremendously exciting scientific experience, but also as a time in which life-long friendships and professional networks were initiated.
 |
| Meyerhof (left) seated beside A.V. Hill, with whom he won the Nobel Prize, at the KWImF around 1931. Standing in the background from left to right are Karl Lohmann, Alexander von Muralt, Grigore Alexandru Benetato, Hermann Blaschko, Arthur Grollman, H. Laser, his technicians Fischer and Schulz and Eric Boyland. Photo: Courtesy of Max-Planck-Institut für Medizinische Forschung |
Research breakthroughs at the KWImF Physiology Institute
By late 1929, it was clear that solving muscle glycolysis would be far more challenging than anyone had imagined. The sheer number of components and the short-lived nature of many of the chemical interactions made the task of sorting out the pathway imposing. Understanding glycolysis was like putting together a giant puzzle. To complicate matters, many of the pieces were still missing from the table. Some that had been found had to be laid to the side until the pieces that fit around them were discovered or put in place. Moreover, it was just becoming clear to Meyerhof that some of the chemical components had been forced into the wrong portion of the puzzle, confusing the overall picture.
A tremendous amount of patience and man-power over a thirty year period had already been focused on this one scientific problem simply to allow a vague outline of glycolysis to emerge. As in any puzzle, however, the discovery of a few key components can lead to a rapid series of related discoveries. This was certainly the case in November of 1929, after which the pieces of Meyerhof’s puzzle began to fit into place at an accelerating pace.
When Meyerhof arrived at the KWImF, he still emphasized thermodynamics and enzyme chemistry in studies of striated frog muscle, but his analysis and methodology were evolving and growing in scale. As data mounted, he and his assistants began to explore the sequence of minute chemical interactions of glycolysis at a much deeper level. Moreover, while his early model – the lactic acid cycle -had led to significant new discoveries, these new discoveries were now forcing Meyerhof to reformulate his famous theory. Meyerhof and his students’ experimental approach at Heidelberg contributed greatly to this success. They were unusually accomplished at breaking down glycolysis into its many separate components, analyzing each step separately, then reassembling the constituent parts within an overall system. Their thermodynamic studies of a wide range of phosphorylated compounds were particularly important at the beginning of the Heidelberg years, while identification and analysis of intermediate products such as esters and the enzymes that catalyze the biochemical reactions became increasingly important during the latter years. Meyerhof also included many comparative studies of intermediate metabolism in other muscle types, animal tissues, as well as fermentation in yeast and bacteria.
Meyerhof and his colleagues at the KWImF remained squarely at the center of this field of research during this dynamic period of discovery. It should be emphasized, however, that other scientists also made important contributions. Parnas, Neuberg, Warburg, Needham, Wieland, the Coris, Embden, Fiske, and others made discoveries that cannot be detailed here. In fact, Meyerhof’s continuing success during the 1930s was partially due to his lack of arrogance about his own ideas. As we will see, he critically and enthusiastically examined the work of others in the field and was quite capable of altering his own theoretical concepts when new evidence indicated its appropriateness.
Lundsgaard and the demise of the “Meyerhof cycle”
Meyerhof and Hill’s pioneering thermodynamic studies had been the basis for the conclusion that the cycle of lactic acid formation and oxidation were the key events in glycolysis. From 1922 until almost 1934, most scientists believed that energy production and mechanical work in muscle were directly coupled to the production of lactic acid. Indeed, after a much heated debate with Embden, who believed that a molecule called lactocidogen was the energy source for the muscle mechanism, this idea became known in some circles as the Meyerhof dogma. Ironically, the discovery in 1926 of the compound creatine phosphate had already caused Meyerhof to publicly question the certainty of his own theory before most other scientists did. Still, in the absence of a better model (e.g. Embden’s lactocidogen proved not to be the activator), Meyerhof continued to support the general theory of the lactic acid cycle, and much of his group’s efforts during the late 20s continued to be directed toward unravelling lactic acid’s role in muscle glycolysis.
The real turning point in Meyerhof’s adherence to the lactic acid cycle came with the arrival of a letter at the new KWImF from the Danish physiologist, Einar Lundsgaard. Lundsgaard told Meyerhof that he had found muscles poisoned with iodatica acid contracted without the production of lactic acid – if creatine phosphate were added to the solution. The ability to contract without lactic acid seemed to contradict Meyerhof’s famous theory. This surprised and excited Lundsgaard. He included in his letter a preprint of his paper suggesting creatine phosphate’s possible direct involvement in muscle contraction. Lundsgaard also asked if he could come to the new KWImF to test his findings using Meyerhof’s thermodynamic methods. Although Lundsgaard’s data seemed to undermine Meyerhof’s famous theories, he agreed immediately to the Danish scientist’s request.
Due of the close relation between the lactic acid cycle and the transfer of energy in muscle, Meyerhof still believed that this set of biochemical changes was critical to understanding the mechanism of contraction. He was, nevertheless, impressed with Lundsgaard’s initial results. Moreover, recent experiments in his own laboratory by David Nachmansohn also suggested a correlation between creatine phosphate and the speed of muscle contraction. If Lundsgaard’s and Nachmansohn’s results were accurate and related, Meyerhof realized he would have to reformulate his model.
When Lundsgaard arrived at the Heidelberg, Lipmann met him at the railway station and brought him straight to the laboratory. Meyerhof and the two younger scientists began experimenting even before Lundsgaard could unpack. Lundsgaard stayed at the KWImF for over six months in 1930, during which time Meyerhof, Lohmann, Lipmann and Blaschko worked with him to refine and extend his initial work.
The early data at the KWImF initially pointed to the phosphorylation of creatine phosphate as the primary event in muscle contraction and, during the next 18 months, much attention was focused on in vivo and in vitro studies of this compound. Lipmann and Nachmansohn, in particular, concentrated on measuring the breakdown and synthesis of creatine phosphate during muscle activity, carefully correlating rates to energy release, muscle tension and simultaneous lactic acid production. In 1931, Meyerhof formally bid farewell to his once favored view that lactic acid itself plays a role in the mechanism of contraction. The new data left him with no doubt that hydrolysis of creatine was of the utmost importance to the contraction mechanism. Meyerhof now tentatively proposed that lactic acid formation supplied the energy for the continuous resynthesis of creatine phosphate at the moment of contraction.
Meanwhile, the discovery of other phosphorylated compounds, esters and enzymes also contributed to the demand for a theoretical overhaul of glycolysis and related mechanisms of contraction. Although much more needed to be investigated, Meyerhof confidently expressed the hope that in a few more years a working hypothesis would emerge to bring order into the mass of new facts that his group and others were rapidly accumulating.
The discovery of ATP and its importance as universal energy donor
Of course, it was ATP, not creatine phosphate, which proved to be the critical source for free energy in glycolysis. But the road to its discovery and understanding of its critical and almost universal role as energy provider in cellular processes began with and led through the analysis of creatine phosphate.
Creatine phosphate was discovered simultaneously in 1926 by Fiske and SubbaRow at Harvard University and the Eggletons in Hills laboratory in England. Until this time, scientists had largely ignored phosphates as important in metabolism. In addition to spawning Meyerhof’s first doubts about the centrality of lactic acid in glycolysis, the discovery encouraged him to begin measurements of the amount of energy released by the splitting of the bonds of creatine phosphate and other phosphate derivatives. When Gustav Embden isolated adenosine monophosphate (AMP) in 1928, Meyerhof and others began a serious search for other phosphates involved in glycolysis and yeast fermentation.
In late 1928, Kurt Lohmann isolated salts of a new phosphorylated compound from muscle, which he called PP. Karl Meyer, who also worked for Meyerhof, had just found the co-factor related to lactic acid formation while attempting to extract enzymes from frog muscle. Identification of this co-factor was turned over to Lohmann at the direction of Meyerhof. In December, Lohmann identified the co-factor as a combination of Embden’s AMP and PP. The compound was adenosine triphosphate, commonly referred to today as ATP.
Adenosine triphosphate, or ATP, was first discovered in muscles by Otto Meyerhof’s assistant Kurt Lohmann just a few months before the opening of the KWImF in Heidelberg. At the time of its discovery, ATP’s role in the biological processes of organisms, from bacteria to mammals, was not recognized. Its central importance came sharply into focus during the next few years, as Meyerhof and his colleagues in Heidelberg worked out step after step of the glycolytic pathway. Lohmann, who worked with Meyerhof from 1924-37, played the dominant role in discovering and unraveling the structure and function of this famous molecule.
Adenosine Triphosphate (ATP)All living cells must create order within themselves to survive and grow. This is thermodynamically possible only because of a continuous input of free energy, part of which is released from cells to their environment as heat. The ultimate source of energy for living cells is sunlight, captured by plants and stored in the carbon bonds of carbohydrates. Animals get energy by eating carbohydrates and oxidizing them in a series of enzyme catalyzed reactions that are coupled to the formation of adenosine triphosphate (ATP). When the high energy of phosphate bonds of ATP are broken during cellular metabolism, free energy is made available for a wide range of biological activities, including muscle contraction, nerve excitation, membrane transport, as well as the manufacture of proteins and nucleic acids.
|
| Karl Lohmann discovered ATP in 1929. Photo: Courtesy of Max-Planck-Institut für Medizinische Forschung |
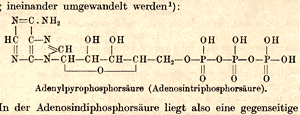 |
| Lohmann’s chemical structure of ATP published in 1935. Photo: Courtesy of Max-Planck-Institut für Medizinische Forschung |
Whether it was intuition, inside information or general practice regarding timely publication, Meyerhof pushed Lohmann to submit his data. It appeared in early August of 1929 in the journal Naturwissenschaften. Although Fiske and SubbaRow had almost certainly simultaneously purified salts of ATP at Harvard University, through an ill-fated decision, they chose not to publish their own results. In late August, Lohmann presented the discovery of ATP at the International Physiology Conference in Boston. Fiske, who attended the lecture, must have sensed his mistake, because he immediately approached Lohmann after the talk and a low-key argument broke out, as witnessed by Severo Ochoa. Fiske and SubbaRow quickly arranged to give an informal talk at the end of the conference and rushed to publish their own data in October. It was too late, however. Priority for the discovery of ATP was awarded to Lohmann.
None of the talks or paper, either by Lohmann or Fiske and SubbaRow, even suggested the huge ramification that this discovery might have for biological processes. In fact, outside of Fiske’s reaction, Lohmann’s presentation at the Physiology Conference evoked little response from those in attendance.
Lohmann’s presentation in Boston of the discovery of ATP came only two months before Meyerhof and his group were to move to the new KWImF. For this reason, there had been no time for the thermodynamic measurements of heat release or structural or functional studies at Berlin-Dahlem. The real meaning of the discovery of ATP would have to wait until follow up work occurred in Heidelberg. This analysis would prove to be a much more daunting and important task than ATP’s initial discovery.
Although Lundsgaard’s work helped Meyerhof’s group recognize the importance of phosphate derivatives, their initial excitement over creatine phosphate pushed analysis of ATP on the back burner for more than a year. Its role surfaced only gradually as Meyerhof’s group patiently broke the glycolytic pathway into its individual steps and analyzed them.
The first hint of ATP’s real importance took place within the thermodynamic studies that Meyerhof had ordered for the various phosphorylated compounds. Lohmann and Meyerhof had begun to group phosphate derivatives into two essential categories – one group characterized by low energy release and one by high energy release upon splitting of their bond. In 1931, Lohmann found that ATP generated considerable heat when split to AMP and two inorganic phosphates. The results placed ATP in the same class of high energy phosphates as creatine phosphate. With these results in hand, Meyerhof encouraged closer examination of ATP, its correlation with other data and interactions with other molecules.
In 1932, Lohmann published an initial chemical structure of ATP. Although he would modify the structure slightly over the next three years, this first paper disproved several prominent competing models and contributed greatly to the momentum of related discoveries at the KWImF. In 1935, Lohmann proposed his refined structural model of ATP, which was confirmed in the late 1940s after the introduction of new X-ray crystallographic techniques.
1932 was also the time Meyerhof’s group first made associations between the uptake of phosphate during the breakdown of carbohydrates to lactic acid and the splitting of ATP. They found that ATP is resynthesized in subsequent reactions. This was the first suggestion that the lactic acid cycle participates in the maintenance of the formation of ATP. By 1934, Lohmann provided direct evidence that ATP synthesis was the by-product of utilization of glucose.
Meyerhof and Lohmann continued to refine their data and analysis. One milestone came in 1934 when Lohmann observed that phosphate molecules from creatine phosphate combine with adenylic acid to form ATP, yet without producing heat. This was the first recognition that high energy compounds could accomplish a biochemical reaction without heat production. These experiments, as well as similar ones by Parnas, pointed to the role of creatine phosphate as a storehouse of energy for phosphorylating ATP. It confirmed and explained the association of creatine phosphate with the speed of muscle contraction that had been suggested by David Nachmansohn’s earlier experiments. Work by Lohmann and Meyerhof, along with important contributions by Parnas, also established the necessity of AMP or ADP in such reactions.
Parnas soon postulated a phosphate cycle, whereby the use of one ATP for phosphorylation is balanced by regeneration of ATP during subsequent steps in glycolysis. If this sounds similar to Meyerhof’s old lactic acid cycle, it is no coincidence. As the structural and thermodynamic data about ATP mounted, combined with the enzymatic information and the discovery of myosin, Meyerhof was finally in a position to formally propose that the release of energy in ATP hydrolysis was the primary event leading to muscle contraction and that lactic acid and creatine phosphate were only indirectly involved through their role of maintaining the ATP cycle.
Of course, we now know that ATP is widely utilized in reactions involving energy transfer in all cells, as well as in bacteria. The discovery of ATP was, thus, the key that opened up the floodgates to understanding many conversion mechanisms of metabolic energy. The unravelling of its structure and bioenergetic role in glycolysis clearly stands out as a major scientific accomplishment.
The role of other intermediate compounds
Although ATP plays a critical role in glycolysis, it is only one actor among many in a very large production. Meyerhof’s greatest achievement was his ability to clarify both the starring and supporting roles of the many molecules involved in glycolysis and then place them in proper sequence of the metabolic script. As research director, this is where Meyerhof’s theoretical insight, holism, openness to new ideas and, indeed, creativity came critically into play.
During the years before Meyerhof came to Heidelberg, a good number of chemical compounds involved in muscle contraction had been found, including lactic acid, pyruvate, methylglyoxyl, hexosediphosphate, AMP, creatine phosphate, as well as several enzymes. That number dramatically increased during the 1930s. The challenge now facing Meyerhof was to identify the specific roles played by each compounds in the complex chain of chemical interactions that lead to the release of energy that drives muscle contraction.
Meyerhof ordered a broad series of tests, especially on esters and enzymes. There were many questions to be answered. For example, what was the chemical nature of each compound? From which other compounds along the pathway did they derive? With which components did each interact in the next step along the pathway? What new compounds were formed or waste products left behind? How did pH and temperature affect the reactions? What enzymes initiated each reaction?
Answering such questions was a challenge because most of the components involved in glycolysis were typified by a very transient existence, making them difficult to isolate, much less analyze, test and then place in proper sequence. Meyerhof ordered experiments with intact muscle, but one must understand that many productive in vivo techniques that we now take for granted, such as using isotopes to tag and trace molecules along the pathway, did not yet exist. Thus, in vitro studies, in which each step along the pathway could be broken down and studied separately, became Meyerhof’s main tool for studying glycolysis. These methods required patience and determination. They sometimes led to experimental artefacts, but their careful application was ultimately the key to Meyerhof’s great success.
One of most important advances of the decade involved painstaking work that helped demonstrate that the formation of esters from carbohydrates is indeed an intermediate reaction in glycolysis. Influential scientists like Neuberg and Harden had found the accumulation of esters like hexosediphosphate during their earlier studies of yeast fermentation, but they had relegated the accumulation of such compounds to the status of a side reaction in the pathway. A few scientists, like Meyerhof, Embden, and von Euler, differed in this view point. Embden believed that the appearance of hexosediphosphate was related to his lactocidogen, while Meyerhof proposed early on that all glucose must go through esterification leading to formation of phosphates. At the time, very little was known about esters and such ideas were quite controversial and not yet backed up by firm data. In fact, the critical role of phosphates in biological interactions remained to be proven to many scientists in the field.
Meyerhof was convinced that an active form of hexosemonophosphate was an intermediate in glycolysis and associated with the formation of pyruvate. But the sequence of steps between formation of these molecules was unknown. Reactions leading to esterification were particularly difficult to identify, because these processes take place at different levels of glycolysis. This picture only gradually became clear as Meyerhof and others simultaneously worked out the cyclical process of glycolysis and the reversibility of certain reactions. It also required the discovery of different enzymes involved in esterification of glucose and polysaccharides and then their purification in order to experiment with the different steps in their in vitro studies. Similar studies on many other compounds and chemical reactions were made throughout the thirties in an effort to fit them into the steps along the pathway.
The unraveling of the system of enzymes
During the 1920s, Neuberg, Warburg and Meyerhof had already broken entirely new ground with direct applications of enzyme chemistry to metabolic studies. Neuberg’s famous work with yeast was probably the birth of the idea that every biochemical reaction is controlled by a specific enzyme. Warburg had became the dominant authority on enzymes in respiration. Meyerhof had managed to isolate and purify the co-enzyme (it turned out to be a complex of enzymes) responsible for conversion of glycogen to lactic acid in muscle. He had then reconstructed the main steps of this complicated set of reactions in cell free solution. He also had applied the same co-enzyme successfully to yeast fermentation, providing earlier proof for the highly similar molecular nature of these two biological processes.
After Meyerhof arrived in Heidelberg, the identification of the individual reaction steps of the metabolic pathways in yeast and muscle glycolysis were increasingly coupled with the study of enzyme mechanisms. Once again, Meyerhof’s group was among the avant-garde of scientists involved in this search. During the next eight years, they discovered more than a third of the enzymes of glycolysis, with eight other research groups combining to find the rest. Lohmann, Kiessling, Schuster, Lehmann and Ochoa were most active at the Physiology Institute in identifying, characterizing and partially purifying these enzymes. Meyerhof’s close relationship to Warburg was of great help here, for it was he who developed new rapid and efficient techniques to purify and crystallize enzymes
| A Chronology of the Identification of the Enzymes of Glycolysis | ||
| Date | Enzyme | Authors |
| 1909 | Alcohol dehydrogenase | Bateilli and Stern |
| 1911 | Pyruvate decarboxylase | Neuberg and Hildesheimer |
| 1927 | Hexokinase | Meyerhof |
| 1933 | Lactate dehydrogenase | Andersson |
| 1933 | Glucosephosphate isomerase | Lohmann |
| 1934 | Pyruvate kinase | Parnas |
| 1935 | Phosphoglycerate phosphomutase | Meyerhof and Kiessling |
| 1935 | Enolase | Meyerhof and Kiessling |
| 1936 | 6-Phosphofructokinase | Ostern, Guthke and Terzakowec |
| 1936 | Fructose-biphosphate aldolase | Meyerhof, Lohmann and Schuster |
| 1936 | Triosephophate isomerase | Meyerhof, Lohmann and Schuster |
| 1936 | Glycogen phosphorylase | Cori and Cori |
| 1936 | Phosphoglucomutase, glucose- phosphomutase | Cori and Cori |
| 1939 | Triosephophate dehydrogenase, Glyceraldehyde-phosphate Dehydrogenase (NADP+) | Warburg and Christian |
| 1942 | Phosphoglycerate kinase | Bücher |
| Table adapted from that of Marcel Florkin in Chapter 24, Vol 31 of Comprehensive Biochemistry. | ||
Early on, Meyerhof’s group spent considerable effort to investigate the effects of hexokinase, which Meyerhof had discovered in 1927. This enzyme rapidly increased the formation of lactic acid and esterification of hexosediphosphate in muscle extracts. Hexosekinase was later found to catalyze the first step of glycolysis by transferring a phosphate group from ATP to glucose to form glucose-6-phosphate. This put an end to the idea of direct phosphorylation of glucose by inorganic phosphate.
In 1932, Lohmann was also the first to detect creatine kinase activity in muscle. This enzymatic reaction is involved in the splitting of creatine phosphate during muscle contraction (the structure of creatine kinase was just recently solved at the MPImF by W. Kabsch in Professor Ken Holmes’ Department of Biophysics). By 1934, Lohmann had experimentally confirmed this important reaction, providing for the first time the description of the mechanism for utilization of phosphate energy. It subsequently became known as the Lohmann Reaction. In 1933, he identified the enzyme responsible for establishment of an equilibrium between glucose-6-phosphate and fructose-6-phosphate. He also found thiamine pyrophosphate to be a co-enzyme, which Severo Ochoa later showed was required for oxidation of pyruvic acid.
Embden-Meyerhof cycle
In the case of glycolysis and alcohol fermentation in yeast, theoretical models and research clearly formed a positive feed-back loop. As in all science, theoretical models are not only an attempt to explain data, but are used to inspire new experimental approaches. Until the mid-thirties, the attempts to test models for alcohol fermentation and glycolysis had more often than not disproved the theoretical constructs. This included, of course, Lundsgaard’s dismantling of Meyerhof’s own lactic acid cycle. Likewise, in 1932, discoveries in the laboratories of Meyerhof and von Euler in Sweden finally undercut the methylglyoxal theory, which had long been favored by many as a way of understanding the pathways in yeast. During the early 1930s, each new discovery of an enzyme or intermediate component led to more data. Working out the importance of the phosphorylated compounds in muscle, for example, greatly stimulated the understanding of energy transformations in glycolysis and changed thinking about how the pathway might be constructed. The understanding of the individual reactions in glycolysis grew geometrically.
By 1932, a cohesive and accurate model of the entire pathway was still clearly needed. Indeed an accurate model for the cycle was just around the corner. It came from one of Meyerhof’s major competitors, Gustav Embden. With accumulating data from his own and other laboratories, Embden constructed a detailed proposal for reaction sequences for almost the entire pathway. The model would prove amazingly accurate. Unfortunately, Embden died in 1933 before he had an opportunity to play a major role in testing the theory.
Meyerhof quickly recognized the great value of Embden’s model. During the next five years, the research groups of Meyerhof, Parnas, Needham, Warburg, Cori, and von Euler effectively worked out the details of glycolysis. Without question, the lion’s share of these reaction steps were analyzed at the KWImF. For this reason, glycolysis has been referred to ever since as the Embden-Meyerhof
| Recognition of the Intermediary Steps of the Glycolytic Pathway | ||
| Date | Step | Authors |
| 1911 | Pyruvic Acid | Neuberg and Wastenson |
| 1928 | Acetaldehyde | Neuberg and Reinfurth |
| 1933 | D-3-Phosphoglyceric acid | Embden, Deuticke and Kraft |
| 1933 | F-1,6-PP | Embden, Deuticke and Kraft |
| 1934 | G-6-P, F-6-P | Meyerhof |
| 1934 | 2-Phosphoenolypyruvic acid | Lohmann and Meyerhof |
| 1934 | Phosphodihydroxyacetone | Meyerhof and Lohmann |
| 1935 | D-2-Phosphoglyceric acid | Meyerhof and Kiessling |
| 1936 | D-Glyceraldehyde-3-P | Meyerhof, Lohmann and Schuster |
| 1936 | G-1-P | Cori and Cori |
| 1939 | D-1,3-Diphosphoglyceric acid | Negelein and Brömel |
| Table adapted from that of Marcel Florkin in Chapter 24, Vol 31 of Comprehensive Biochemistry. | ||
Meyerhof had long been convinced of the similarity of basic molecular processes in all life forms. He had argued as early as the twenties in favour of the unified pathway in yeast fermentation and glycolysis. Although he is most famous for his work involving muscle contraction, he injected considerable energy during the Heidelberg years into comparative studies with yeast. As they delved deeper into the pathways, he and his colleagues found the reactions and intermediates in muscle and yeast cells to be extremely similar. Indeed, much of the critical identification of glycolytic enzymes in Meyerhof’s laboratory was done in connection with experiments on yeast. In 1935, with great assistance from Kiessling, Meyerhof was able to experimentally confirm that with only minor differences, the metabolic pathways in muscle and yeast were indeed the same.
Recognition of the unified pathway of glycolysis represented major progress and was a turning point in silencing those who argued that unity at the molecular level could not exist. In search of further support for the concept of molecular unity, Meyerhof searched for the occurrence of similar reaction patterns in other biological materials throughout his tenure in Heidelberg, including important studies on different types of muscle, as well as bacteria. This led to the discovery of phosphoarginine in the muscle of invertebrates, which serves a similar role to that of creatine phosphate in vertebrates.
The threat of national socialism finally forces Meyerhof to flee Heidelberg
Not surprisingly, the rise of the National Socialism in Germany had a dramatic impact upon the development of the KWImF Physiology Institute. Meyerhof watched painfully as close colleagues and students, like Blaschko, Lippmann, Neuberg, Nachmansohn, Ochoa, Krebs and others made their way out of Germany, one by one. During this period, both the KWG and Krehl encouraged Meyerhof to remain in Heidelberg and continue with his important research. Initially, this support and his prestige as a Nobel Prize winner helped to shield Meyerhof and his family from the excesses of the Nazis. Like so many others, Meyerhof was convinced that the National Socialists were unlikely to maintain their grip on power and, because his work at the Physiology Institute was proceeding so marvelously, he chose to remain in Germany – dangerously late as would become clear in retrospect.
It was not until 1937 that Meyerhof began making secret plans to leave the country. Writing in code, his former assistant David Nachmansohn arranged for a position in France for his old professor. Having already sent his two older children abroad, Meyerhof and his wife Hedwig received special permission in 1938 to pass into Switzerland for medical treatment of their youngest son. Once across the border, they then made their way safely on to Paris. To protect the deception, Meyerhof told none of his colleagues of his departure. Unfortunately, this also meant he was forced to leave behind all of his scientific data and personal possessions. Two years later, the German invasion of France sent the Meyerhofs on another harrowing journey across the Pyrénées and Spain to Lisbon, where they boarded a ship bound for Philadelphia in the USA. Meyerhof worked at the University of Pennsylvania until his death in 1951.
The influence of the Meyerhof school




Fritz LipmannSevero OchoaAndré LwoffGeorge Wald
Otto Meyerhof was not only an important figure in the building of the foundations of modern biochemistry, he also trained a large number of scientists who went on to carve out outstanding careers in their own right. In addition to scientists like Kurt Lohmann, David Nachmansohn, W. Kiessling, and Paul Ohlmeyer, those working with Meyerhof at the KWImF included four future Nobel Prize winners: Fritz Lipmann, Severo Ochoa, André Lwoff and George Wald. These scientists spread out across the globe during the 1930s, helping to bring about a wave of discovery in the emerging field of biochemistry. By their own accounts, each was decisively influenced by Meyerhof’s scientific ideas and his personality and maintained close contact with Meyerhof until his death in 1951.
Lipmann left Heidelberg for Denmark, finally settling in the USA at the start of WWII. In the early 1940s, Lipmann extended our understanding of bioenergetics by formulating the concept of the ATP metabolic wheel and introducing his famous “wiggle” (~P) to represent the bonds of high energy phosphate derivatives. In 1953, he was awarded the Nobel Prize for his discovery of co-enzyme A and its importance for intermediary metabolism.
Severo Ochoa spent two different periods at the KWImF – first in 1930-31 and again in 1936-37, during which he analyzed enzymatic steps of glycolysis and fermentation. He later worked with the Coris and, like Lipmann, settled permanently in the United States. Ochoa’s later research dealt principally with enzymatic processes in biological oxidation and synthesis and the transfer of energy. In 1959, he was awarded the Nobel Prize for discovery of the mechanisms involved in the biological synthesis of ribonucleic acid.
While working with Meyerhof in Heidelberg, André Lwoff studied haematin – a growth factor for the flagellates – the specificity of protohaematin, its quantitative effect on growth, and the part it played in the respiratory catalyst system. Lwoff settled in Paris in the late 30s, where he began studying bacteria and viruses. In the 1950s, he joined Jacques Monod and François Jacob in building the foundation of French molecular biology. Together, they were awarded the Nobel Prize in 1965 for their discoveries concerning genetic control of enzyme and virus synthesis.
George Wald first identified vitamin A in the retina while working with Otto Warburg in 1930. Vitamin A had just been isolated in the laboratory of Paul Karrer in Zurich and Richard Kuhn at the KWImF. At Warburg’s suggestion, Wald moved on to the KWImF to take advantage of the creative insights of Meyerhof and Kuhn. Wald returned to the United States in 1934. Thirty-three years later, he won the Nobel for his discoveries concerning physiological and chemical visual processes in the eye.

Otto Meyerhof
Photo: Courtesy of Walter Meyerhof, son of Otto Meyerhof
As a youth, Meyerhof’s relationship with his mother was particularly close. It was she who encouraged him to harness his intellectual abilities. Meyerhof quickly demonstrated a broad range of interests that included music, art, natural sciences, history and architecture. He became an accomplished pianist, wrote poetry and soon developed a passionate interest in the philosophy of Kant and Fries. A later attraction to quantum physics was based as much in his fascination with the epistemological questions raised by Einstein, Heisenberg, Bohr, as it was for scientific considerations.
Meyerhof demonstrated an early professional tendency toward multidisciplinarity. Although he studied medicine, his humanistic interests were reflected in a dissertation on a topic in psychiatry. And during his career in biological research, he was largely successful because of his ability to integrate chemistry, physics and physiology into a single cohesive approach.
Anti-Semitism had a profound effect on Meyerhof’s professional and personal life, even before Hitler came to power. Nevertheless, Meyerhof’s decision to flee the country in 1938 weighed heavily on him. Meyerhof may have been Jewish, but Germany was his homeland. Moreover, as he feared, he was never again provided with the rich scientific resources that were at his disposal at the KWImF. The years that followed immediately were depressing times for Meyerhof. Although he, his wife and children escaped safely from the holocaust, the family lost almost all of its possessions, including Meyerhof’s beloved library. And yet, Meyerhof continued to draw strength from his philosophical approach to life and his professional career, in which he remained active until his death in 1951.
In retrospect, one is struck by the dignity and generosity Meyerhof demonstrated towards others throughout his career. Assistants and competitors alike found that he listened closely and respectfully to their ideas, whether he agreed or not. To his assistants and students in Heidelberg, he was both a mentor and true friend – an unusual practice, given the elevated stature of senior scientists and the formality of the time.
The Personality of MeyerhofOtto Meyerhof was born in 1884 in Hannover, Germany. His father had come from a small Jewish enclave in the nearby city of Hildesheim – notable largely because the Hildesheim Meyerhofs had extensive kinship relations with the families of two other scientists who knew Meyerhof well and became fellow pioneers of modern biochemistry – Hans Krebs and Carl Neuberg. |
