Albrecht Kossel
Nobel Prize lecture
Nobel Lecture, December 12, 1910
The Chemical Composition of the Cell Nucleus
The development of organic chemistry in the past century has been based chiefly on the building up of concepts as to the arrangement of the atoms in space. As you well know, the organic chemist is able to present both this knowledge of the composition of an organic substance and its chemical reactions, and his views on its position in the chemical system, with clarity and precision by constructing a picture which shows the distribution of a number of various kinds of atoms in space.
When one uses these conceptions in the investigation of animal or vegetable tissues one is led to the picture of a chemical structure of these organic formations. The scientific domain which is opened up in this manner can in many ways be compared with the science of the anatomical structure of living beings.
Both disciplines, the anatomical and the chemical, appear at first to strive only for the description of an organic formation, but in both cases this description is really not the true aim of the investigation. This knowledge of the anatomical as well as of the chemical structure is only valuable in our view because from it we hope to gain understanding of the function of the various parts, or of the mechanism of their development, or other questions of biological importance.
Accordingly we can only evaluate experience on the composition of the cell and the protoplasm as preliminary steps to greater knowledge. The results of these obtained so far, which I shall attempt to report, are really more suitable to stimulate our thirst for knowledge rather than to gratify it. There is still a long way to go from a consideration of individual fragments of the apparatus to an understanding of its mode of action.
Comparative observations have led to the conception that there are certain chemical vital processes common to animals and plants, that there is to some extent a chemical mechanism which acts on common principles in the various kinds of living matter. These basic physiological processes must be situated in that substance which appears everywhere to be the main focus of the physiological combustion processes, from which, at the same time, the remaining parts of the body proceed, i.e. the protoplasm.
Obviously the chemical investigation of this structure must seem to be one of the most important problems of biochemistry, but the difficulties of such an investigation became apparent in the first analyses – first of all in the selection and preparation of the material for them. The living cell almost always contains, locked in its interior, the visible or invisible products of its physiological activity or its nourishment. The distinction between components and inclusions, between organized body substances and chemical metabolites is hard to define and results definite to some extent can only be expected on the basis of careful histological appraisal and comparative study. Thus the most varied cell structures and formless protoplasms have been investigated and individual groups of compounds defined which repeatedly emerge in the lists of these components, and since Hoppe-Seyler’s work nucleins, lecithin, cholesterol, and finally potassium salts, in addition to proteins, have been added to the list.
New prospects were opened up when attempts were made to bring the cell nucleus within the scope of these investigations. Here we have an organ of the cell whose structure and function must be associated with the general processes of life. This is already evident from structural conditions, and from the changes in form which precede and accompany the processes of cell-division, which recur in different regions of the animal and vegetable worlds and are fundamentally unrelated to species and group or to the position of the system in the organic world. Chemical characteristics have now been added to the morphological characteristics of this organ which define its peculiarities still more sharply because they can also be recognized in cells in which the structure of the nucleus is not defined. These chemical characteristics I shall now briefly attempt to outline.
The first observations in this sphere were begun in Hoppe-Seyler’s laboratory in the 1860’s on the nuclei of pus cells. Miescher, a student of Hoppe-Seyler’s, was able to isolate these nuclei and he found in them a substance very rich in phosphorus which he called “Nucleïn”. A suitable object for the carrying further of this work was found in a structure which develops through the transformation of the cell nucleus and which retains its chemical constitution and apparently also an essential part of its physiological function – namely in the heads of spermatozoa. In the course of the next decades evidence accumulated to show that “Nucleïn” or “nucleic material” is indeed peculiar to the cell nucleus. Still other objects were found which to some extent permitted the isolation of the cell nuclei, e.g. the red blood cells of birds where the cell body is soluble in water. Here also chemical investigations could be carried out on adequate masses of nuclei thus isolated and again the distinguishing features of nucleic material were found, and microchemical tests confirmed this. They showed at the same time that the nucleic material belonged to a well-defined part of the nuclear substance which stood out in a very conspicuous manner during the transformation process, whose amount in various nuclei is variable and which because of its reaction to certain stains has received the name of “chromatin”. There was only one difficulty about this which was the finding of “nucleic substances” in animal products which contained no cell nuclei, namely in the yolk sacs of eggs and in casein in milk, and indeed attempts were made to explain these facts by special hypotheses before more accurate chemical investigations brought clarification.
The chemical structure of these nucleic substances shows some peculiarities which are found in many organic components of protoplasm, particularly in those which participate actively in metabolic processes. It has been observed that such components break down easily into a certain number of closed atom groups which have been compared to building blocks. Such “building blocks”, fitted together in great number and variety and apparently according to a definite plan, form the molecule of proteins, and also of starch and glycogen, and in smaller numbers those of fats and phosphatides. The complicated organic components of nutrition are broken down into these building blocks when they are prepared by digestion for being taken up into the body, and from these building blocks the large molecules inside the organism can then again be fitted together.
The nucleic substances show a composition of this sort also. Chemical analysis showed first that in many cases nucleic substances are broken down into two parts, one having the character of a protein. This part possesses no other atom groups than the usual proteins. The other part, however, is of characteristic structure; it has been given the name of “nucleic acid“. From it I succeeded in obtaining a number of fragments which could be dissolved out of the molecule in part even by gentle chemical action and which were recognizable by a quite special concentration of nitrogen atoms. Here four nitrogen-containing groups are present together: cytosine, thymine, adenine, guanine.
One of these four bodies, guanine, has been known for some time in various animal tissues and was found by Piccard for example in the spermatozoa of salmon, although indeed this investigator had no suspicion that it had any genetic relationship with the nucleins. Earlier it was generally accepted that guanine and other similar substances originated from the protein molecule and Miescher thought that these bodies perhaps arose from protamine, while Piccard put forward the idea that “they pre-existed along with it in the salmon sperm”. Knowledge of their origin from nucleic acid, which was unexpected and, to start with, ran up against active opposition, gave at the same time an understanding of particular phenomena for which an explanation had been lacking; e.g. it had been noted that in leukaemia, guanine and related substances were present in large amounts in the blood. Now in this disease it is typical that the non-nucleated red cells are replaced by elements containing nuclei, but these latter break down in large numbers and accordingly the body fluids are overwhelmed with the decomposition products of nucleic material. Hence the bases just mentioned, or their very closely related transformation products, occur in large amounts in the body fluids. Also the contradiction mentioned previously, which appeared in the supposed presence of nucleic material in egg yolks and in milk, was now solved. A more accurate investigation showed that these elements, which because of their external behaviour and phosphorus content had previously been considered as nucleins, possess a chemical structure of different type. The nitrogen-rich building blocks as I have called them, are completely absent – thus they really do not belong to the group of the true nucleic substances and form a special class.
The more the relationships of the nitrogen-rich substances to the cell nucleus were recognized the more the question of the arrangement of the nitrogen and carbon atoms in the molecule came to stand out. Two of the named four bodies, adenine and guanine, belong to a group of chemical compounds which today are usually included under the name of alloxan derivatives or purine derivatives. The discovery of the individual members of this group and the elucidation of their chemical nature is linked with the names of Scheele, and Torbern Bergmann, Wöhler, Liebig, Strecker and Adolf Baeyer and the brilliant series of these investigations was concluded with the work of Emil Fischer which led to the satisfactory final establishment of the formulae shown below. The two others, thymine and cytosine, showed a simpler composition; experiments of breakdown and synthesis led to the result that in thymine there must be a grouping of the carbon and nitrogen atoms corresponding to the following scheme:


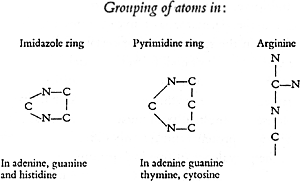
It is evident from the formulae that in thymine and cytosine a ring-like system of carbon and nitrogen atoms must be assumed. The position of the atoms in cytosine could be determined, because under the action of an oxidizing agent this substance breaks down into biuret and oxalic acid, and this elucidation of its constitution was soon followed by its synthesis. In contrast to this simple ring which is known as a “pyrimidine ring”, there is in the formulae for adenine and guanine a double ring, the so-called “purine ring” which shows a still greater concentration of nitrogen atoms.
In these four fragments of the nucleic-acid molecule the carbon and nitrogen atoms are seen to fit together according to the same basic plan. The purine ring arises as it were as a result of a structural addition to the pyrimidine ring. If now the known four pyrimidine and purine derivatives are exposed to stronger chemical action or if their behaviour is followed in the body, it can be seen that those carbon and nitrogen atoms whose linkage permits the formation of the ring are rather difficult to separate from one another and that in contrast, other atoms which are additionally attached to the ring, for example the NH2 group, can be detached by the introduction of the elements of water. In this manner derivatives develop which are called hypoxanthine, xanthine, and uracil and which at times are found alongside of adenine, guanine, and cytosine, and furthermore still other substances which appear as end products of animal metabolism.
Hence now we are clear to a certain extent about a part of the nucleic-acid molecule – i.e. the nitrogen-containing portion; but there is still a remainder which is made up of two dissimilar components. One of these contains 6 carbon atoms which are linked with oxygen and hydrogen in the manner characteristic of carbohydrates; the other which contains no carbon is phosphoric acid.
If the nature of the individual building blocks in such a large molecular structure as is present in nucleic acid has been ascertained, two new questions arise: What are the relative amounts of each block, and how are they mutually arranged? The first of these questions has been answered by the investigations of H. Steudel. According to his analyses we have to assume that for each of the four nitrogen-rich groups there is 1 carbohydrate molecule and 1 of phosphoric acid. At the present time the second question cannot yet be answered adequately. There is only one observation which allows the conclusion to be drawn of an association between the carbohydrate group and the nitrogen-rich bodies, i.e. both fragments, if the nucleic acid is carefully broken down, are still found linked together and also occur in this combination in the metabolism of plants.
According to this fleeting survey of our present knowledge and opinions, nucleic acid appears as a complex of at least 12 building blocks, but in the living cell the structure is probably larger, because some observations suggest that in the organs several of these complexes are combined with each other.
I have attempted to give a description of one nucleic acid, which is contained in certain cells of the animal organism, but this is not the sole form in which representatives of the nucleic-acid group appear. Investigations of various organisms and various organs of the same individual have demonstrated an important diversity in the structure of this class of substances. The same phenomenon is repeated in the nucleic acid which we know in proteins, fats, bile acids and many other biochemical products – the development of a whole series of various kinds of substances which show the same architectural idea carried out in many and varied ways.
The structure I have outlined of the nucleic acids is repeated in other organs in a simpler manner. For example, a nucleic acid is found, in the cells of yeast, which lacks thymine, one of the four nitrogen-containing groups, and which instead of the 6-membered carbohydrate ring contains one with 5 members. The composition of inosinic and guanylic acids is still simpler. The first-named was already discovered by Liebig, though Haiser first recognized its chemical nature, and occurs in muscle and contains in the place of the four nitrogen-containing substances only a single one and this in a somewhat altered form, and also a carbohydrate with only 5 carbon atoms. A similar structure must be ascribed to guanylic acid, a substance which was first discovered by Olof Hammarsten and Ivar Bang. Here also there is only one nitrogen-containing group, in this case guanine, and here also there is a 5-membered chain of carbon atoms linked as a carbohydrate with guanine and phosphoric acid.
It is quite understandable that the interest of biochemists has been drawn to these substances since they have become recognized as the simplest members of the nucleic-acid groups. This knowledge is still recent; research first of all made its way through forms intricate and difficult to recognize, before it succeeded in getting a hold on forms, which are simple and easy to grasp. We do not know whether inosinic and guanylic acids are of the same importance to the life of the cell as the complicated nucleic acids; at present in particular it has not been established if the site of the two last-named acids is to be sought in the chromatin of the cell nucleus.
As I have previously mentioned the complex nucleic acids are found in this morphologically so important structure in combination with “proteins” and these combinations can occur in a large variety of ways. In some organs a loose combination of these two components is found which behaves like a salt, and from which both the acid and the protein can easily be isolated. In other cells there is a firm combination between them which is strongly resistant to the action of chemical separating agents. The salt-like form is found in the nuclei of erythrocytes in the blood of birds and as I have already stated these nuclei can be isolated if the red cells are dissolved in water. The substance of the cell nucleus then remains behind with some of the associated “stroma”, as an insoluble mass. If this mass of nuclei is brought into contact with dilute acids the greater part of the protein is dissolved while the nucleic acid is left. Similar loose combinations are found also in cells of the glandular tissues: thymus, lymph glands, and the spleen, and in all these tissues it can also happen that one part of the proteins is present in a firm, another in a loose combination. The behaviour of the heads of spermatozoa, which in their origin and histological characters really belong to the cell nuclei, is note-worthy. It might be assumed that in an organ which possesses one and the same function in various animal species, similar chemical relationships will be found, but this is not the case as regards the protein-nucleic acid combination. In investigations, which however have so far only been performed on a small number of species of warm-blooded animals, it has been found that in the spermatozoa of warm-blooded animals there is a firm combination as against a loose one in invertebrates – possibly in many cases along with a firm one. Fish spermatozoa behave like the nuclei of erythrocytes in birds’ blood in which so far only a loose combination has always been found, though whether a firm one is also present has not yet been decided.
The nuclei in which there is a loose combination of nucleic acid show another notable phenomenon: namely, a special disposition of the proteins which are combined with the nucleic acid. These have the character of an organic base. The nuclei in which the protein is firmly combined are much less susceptible to chemical investigation and will be omitted in the following description.
In order to make the transformation of the protein molecule to a base comprehensible, I shall try briefly to bring out the most essential peculiarities of the chemical structure of these class of substances which are so important for the organic world.
Proteins, like the above-mentioned carbon compounds of the cell, are made up of a large number of connected groups, the so-called “building blocks”, by which I mean here a complex of directly connected carbon atoms. Where the linkage of these carbon atoms is interrupted by other atoms, a detachment of these blocks usually occurs when the large molecules break down in or outside the organism. The number of carbon atoms which are encountered in direct and firm combination in these large building blocks of protein amounts – so far as has been ascertained with certainty hitherto – to a maximum of 9, possibly 12, but in most cases the groups are smaller. The uniting of these groups with one another is usually induced by a nitrogen atom, which at the same time is linked with a hydrogen atom, and forms a so-called “imid” group. This method of linkage has been determined mainly by the work of Emil Fischer. In special cases other methods of linkage may well occur, e.g. the disulphide linkage discovered by E. Baumann in which the connection of two carbon chains is made by two interlinked sulphur atoms. This occurs in cystine, which is known from the work of Count K.A.H. Mörner as a component of the protein molecule. If now the protein molecule is broken down, this usually comes about with the introduction of the elements of water.
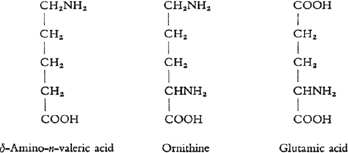
At least 19 different species can be distinguished among the building blocks which thus release themselves from the structure of the whole molecule. The majority of these building blocks or fragments are dominated as far as the inner structure is concerned by one common principle. Nearly all these fragments have the characteristics of an “amino acid”. As an example of such a substance one can put forward amino-valeric acid. This contains a chain of carbon atoms to which hydrogen, oxygen and nitrogen atoms are attached. The first characteristic of these substances is the COOH group which bestows on them the qualities of an acid, the second is the NH2group whose presence induces the qualities of a base. Now we know of amino acids which as in the example given of delta-amino-eta-valeric acid contain an equal number of COOH and NH2groups; also others in which there is one NH2group more, and still others which contain one COOH group more. In the latter the acid qualities, and in the former, the so-called diamino acids, the basic qualities predominate.
 |
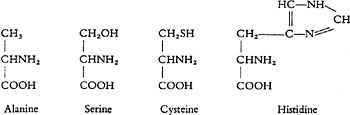
The multiplicity of the protein-forming amino acids is however not solely due to these variations in the number of the COOH and NH2groups, but also to the number of the carbon atoms which are linked in a chain. We can obtain chains with 2, 3, 5 or 6 carbon atoms from the protein molecule, and further variations may be brought about by the separation of one of the hydrogen atoms from the carbon atom through introduction of an atom of oxygen or sulphur, or because a complicated organic group, for instance with 3C, 2N, and 3H, takes the place of one H atom.
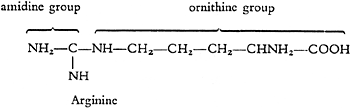
As well as these amino acids a quite different kind of atom group is found in the series of protein-forming building blocks, which contains one carbon atom and two nitrogen atoms and in the molecule this group is always in combination with the diaminovaleric acid mentioned previously. The combination of this “amidine group” with diaminovaleric acid, or omithine, which is named “arginine” was discovered by E. Schulze and demonstrated by S.G. Hedin to be a component of protein.
 |
Thus the protein molecule is made up from such building blocks. We do not know how often each block is repeated in the whole structure but we are able to determine the relative proportion between the amounts of the variously formed building blocks. For example, we can determine how large the amount of the diamino acids is compared with the monoamino acids and what percentage of the total nitrogen is present in the form of diaminovaleric acid. Already these ratios, though they do not give any idea of the relative arrangement of the blocks, have shown quite important differences between the proteins investigated hitherto, and further they show that among them, the previously mentioned loosely bound proteins of the cell nucleus occupy a quite special place.
The peculiarity of these nuclear proteins depends on the fact that larger amounts of certain kinds of building blocks, namely the nitrogen-rich groups, are concerned in their construction. Thus for example in comparison with the remaining proteins they contain larger amounts of diamino acids, especially diaminovaleric acid, and the amidine groups attached to it; histidine can also occur in them in large amounts.
The insertion of these nitrogen-containing group in the protein molecule is moreover such that strongly basic groups are present in the freely reactive state.
A protein of this kind is found for example in the nuclei of birds’ erythro-cytes and as I have already mentioned may be easily removed by dilute mineral acids. It is called “histone”. Similar substances are widely distributed in the tissues of higher and lower animals in a salt-like combination with nucleic acid. They also occur in the spermatozoa of invertebrates, e.g. the sea-urchins, Cephalopoda, and also in the spermatozoa of certain fishes. As an example of this I can quote various kinds of cod, from the testicles of which we could obtain a histone which is very similar in its chemical qualities and composition to the histone obtained from the erythrocytes of birds or from the thymus.
These histones, loosely combined with nucleic acid, thus show the nature of the usually complicated proteins and are only differentiated from them by one special quality, the prevalence of free basic groups.
If the testicles of other fish are submitted to the same investigation, bodies of a much simpler composition are obtained which take the place of the histone in the heads of the sperms; these are protamines.
The opinion has been formed from a whole series of observations which I shall not mention here that these basic proteins have arisen in the course of development through transformation of the ordinary proteins in that the groups poorer in nitrogen have gradually been dissolved out of them. This transformation can be more or less extensive. It leads from the ordinary proteins, first to the histones, and if the elimination process is carried on still further we arrive at the protamines. These are thus still poorer in monoamino acids and relatively richer in diamino acids than histone. But the protamines also differ from one another and are obviously linked by intermediate steps with the histones. The sturine obtained from sturgeon roe contains for ex-ample all four of the previously mentioned nitrogen-rich groups of the protein molecule: two diamino acids of which one is combined with the amidine groups, and in addition, histidine. Other protamines contain only two or three of the known basic groups. The variety in the composition of the protein molecule is notably reduced in the heads of sperms of certain Salmonidae, and here the whole molecule is limited to 5 different kinds of building blocks. Two of these, diaminovaleric acid and the amidine group are the main carriers of the nitrogen and predominate in quantity over the remainder, carrying about 88% of the total nitrogen.
Thus in this peculiar transformation more and more of the long nitrogen-poor carbon chains which are so essential in the build-up of most proteins that they form the main part, disappear, and against this a group becomes evident which shows C and N in an alternating arrangement. This arrangement is present also in another component of the cell nucleus, namely nucleic acid, as we have seen.
If we now summarize the results of the investigations of loosely bound nuclein substances, the result is a follows: A composition of the chromatin substance of the cell nucleus from two components, the one rich in bound phosphoric acid and having the qualities of an acid; the second showing a protein with the qualities of a base. In their chemical structure both components show a notable similarity based on the remarkable accumulation of nitrogen atoms and because of this chemical structure the chromatin formations can be sharply differentiated from the remaining cell components; and this quality must obviously be associated with the function of the chromatin substances. These atom groups rich in nitrogen and containing phosphorus are those whose deposits in the chromosomes are first set in motion during cell division and whose transmission to other cells forms an essential part of the reproductive process.
At this point we have arrived at problems the solution of which can only be attained by various methods of research working together. The representatives of morphological sciences see under the microscope a structure deposited in the cell and study the dependence of its form on the conditions of the elementary organism. The biochemist tries to define the composition of this structure, its position in the chemical system and at the same time its relation to other chemical components of the cell, but this task demands theories of structural chemistry and the aid of synthetic methods.
Thus the results which I have attempted to portray today have originated from various research institutions, and the names of many people would have to be mentioned if the merits of all contributors were to be acknowledged.
The Nobel Foundation's copyright has expired.Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
