Press release

NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
10 October 1980
The Nobel Assembly of Karolinska Institutet has decided today to award the Nobel Prize in Physiology or Medicine for 1980 jointly to
Baruj Benacerraf, Jean Dausset and George Snell
for their discoveries concerning “genetically determined structures on the cell surface that regulate immunological reactions”.
Summary
The surface of all body cells is unique in every individual. This unique character is determined by genes that regulate the formation of specific protein-carbohydrate complexes (MHC) – the histocompatibility antigens, or H antigens – found on the cell membrane. These complexes derive their name (histo denotes a relationship to tissue) from the fact they define the capacity of a body tissue to exist in intimate contact with another body tissue. H antigens determine the interaction among the multitude of different cells responsible for the body’s immunological reactions. Knowledge of the genetic regulation of the body’s immune response makes it possible to explain why different individuals have different capabilities of defending themselves against infections and why a cancer cell is eliminated in some cases and enabled to grow into a tumor in others. The genes that are important in this connection have been demonstrated primarily in studies on mice and humans, but they are found in all vertebrates. Knowledge of H antigens is of great practical importance, for example, in tissue transplantation (the transfer of tissues from one individual to another) and for understanding the relationship between the genetic constitution and disease. Thus, it has been shown that certain H antigens predispose certain individuals to certain diseases.
George Snell discovered the genetic factors that determine the possibilities of transplanting tissue from one individual to another. It was Snell who introduced the concept of H antigens.
Jean Dausset demonstrated the existence of H antigens in man and elucidated the genetic factors regulating their formation.
Baruj Benacerraf showed that genetic factors intimately related to the genes that determine an individual’s unique constitution of H antigens actually regulate the interaction among the various cells belonging to the immunological system and are thereby important to the strength of an immunological reaction.
Every individual is unique. This is true even with regard to the many details of the body’s structure. An important example are the antigens (protein-carbohydrate complexes) found on every cell membrane in the body. Through studies on the relatively simple red blood cells, for example, it has been possible to demonstrate the presence of different blood group antigens. The formation of the antigens found in the cell membrane is regulated by information found in the cell’s genes. Thus, the characteristics of the individual, as well as of his cells, are genetically determined.
If cells with different surface properties come into contact with each other within an organism, which is what happens in the case of tissue transplantations, a reaction takes place that results in the foreign cells being rejected by the body. Transplantation, however, is an artificial situation that does not normally occur in nature. (An exception is the case of the pregnant woman. The cell membranes of fetal cells contain antigens determined by the genes inherited from the father.)
Therefore, the immunological reaction causing rejection of a foreign transplant must exist in order to fill some other function. This function is to see to it that the body’s cells do not alter their unique surface characteristics. A change in these characteristics can occur, however, in connection with a virus infection or when a normal cell is transformed into a tumor cell. It is in this situation that the body’s ability to distinguish “self” from “non-self” takes on its great significance. It is essential that this defense system is carefully balanced so that the body does not suddenly react against its own normal cells. If this were to happen, a so-called auto-immune disease would arise.
The genetic control of the body’s immunological reactions also plays a decisive role in the body’s defense against infectious agents. People show varying ability to mobilize resistance against infections, and to a large extent an individual’s ability to react would seem to be genetically determined.
George Snell has laid the foundation for our knowledge of the laws that govern the body’s ability to distinguish “self” from “non-self”. He did this by studies on mouse strains that, through repeated sibling mating, were made as genetically identical as monozygous twins. Originally, he studied the possibility of transferring tumor cells from one strain to another. The rules of transplantability that he established proved to apply also to normal tissue, such as skin. Snell showed that transplantability was determined by the presence of special structures (antigens) on the surface of the cell. He called these antigens histocompatibility antigens. He showed, further, that the formation of these antigens was controlled by genes (designated H) found within a limited area on a specific chromosome. This area was called the major histocompatibility complex (MHC). So far it has been possible to establish the existence of about 80 different genes within the MHC in mice. With Snell’s fundamental discoveries came the birth of transplantation immunology.
Between 1930 and 1950 when knowledge about transplantation immunology was increasing in the mouse, nothing was known about a corresponding system in man. Experimental tissue transplants comparable to those practiced on laboratory animals were not possible. Nevertheless, Jean Dausset’s research was to dramatically blaze the trail to studies of rules for transplantation in man. Originally, Dausset studied auto-immuno diseases, and one of his methods was through immunological investigations of patients who had undergone repeated blood transfusions. The antibodies found in these patients proved to have no significance at all when it came to auto-immune diseases, but were instead an important indicator of differences in the cell-membrane structure of white blood cells between blood donors and recipients. When he went on to study the antibodies of women who had given birth to several children, Dausset was able to show that one single genetic system, localized on one single chromosome, determined these antigens. They came to be called human leukocyte antigens (HLA), and the genes that determined their formation, HLA genes (Figure 1). Thereby Dausset had identified the human equivalent to the H-genes in mice. The similarities between the two systems were soon shown to be much greater than originally suspected. Dausset showed that within the HLA system in man there were two dominating regions, and Snell was subsequently able to show that this was the case, too, with the H system in mice. All species that have so far been studied (from among reptiles, fish, birds and mammals) have been shown to have an MHC.
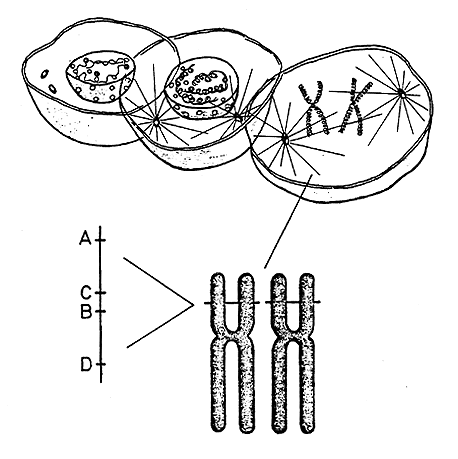 |
Figure 1. The genetic material (DNA) is located in the cell nucleus in the form of long threads – chromosomes – that contract and can easily be studied during cell division. Humans have 46 chromosomes in each cell and these can be divided in 23 pairs which are numbered from 1 to 23. In other words, each chromosome exists in duplicate. (The illustration shows only one chromosome pair). On a tiny area of chromosome number 6 are found a large number of genes (MHC=major histocompatibility complex) that regulate, among other things, various immune reactions and the development of tissue antigens (HLA). HLA antigens are regulated by four different genes (A, B, C and D) each of which can occur in many alternative forms. A has at least 15 variants, B 29, C 9 and D 12. Every human can have at most two variants of each gene – one on each chromosome number 6. The probability for two unrelated people having exactly the same constitution is small since there are more than 100 millions possible combinations. Monozygote twins always have the same constitution of HLA antigens.
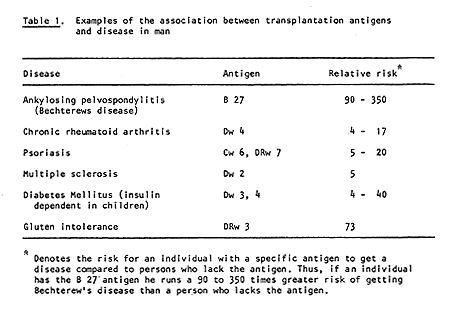
Dausset’s discovery has many practical applications. With the aid of his system it is possible to type both donor and recipient in cases of kidney transplantation. Thereby the possibilities for a successful transplant are considerably increased (Figure 2). HLA typing has also been of great importance in the investigation of disputed paternity and in anthropological and evolutionary studies, i.e., comparison of groups of individuals belonging to different species and races. It has even been possible to H type mummies. It has also been found, somewhat unexpectedly, that persons with certain HLA antigens run a significantly greater risk of getting certain diseases than persons who lack those antigens (Table 1). The reason for this is still unclear. The portion of the genetic material that determines the formation of transplantation antigens (MHC) also includes hundreds of other genes, and one or several of these may be significant in this connection.
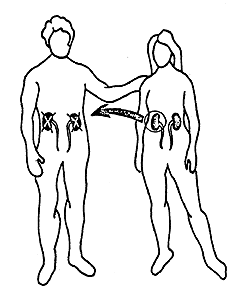
Figure 2. 90 % to 100 % of all kidney transplantations between siblings with the same HLA antigen constitution are successful. The corresponding figure for siblings or for parents and children with partially different HLA antigen constitution is 70 % to 80 %. In cases in which a patient receives a kidney from an unrelated cadaver the possibility for a successful transplantation is about 50 %. In Sweden about 200 kidneys are transplanted each years; in the world about 10,000.
 |
A very important group of genes within the MHC has been demonstrated by Baruj Benacerraf. He has shown in elegant studies that the guinea pig’s ability to mobilize an immune response against a certain antigen is determined by genetic factors. He called these factors Ir (immune response) genes. Several of these genes have been identified and found to be located within the same chromosome region that determines the formation of H antigens. Thus, this region has several very central functions for regulating various immunological reactions in the body. This is why the MHC has come to be referred to as a “super gene”. The area of research opened up by Benacerraf now offers the possibility of analyzing the background of the varying ability of different individuals to mobilize an immune response to infections. Among the problems to be considered here are cell interaction, cell identification and the activation of immunological reactions.
Reference material
Cunningham, B.A.: The Structure and Function of Histocompatibility Antigenes. Scientific American 1977, 237, 96-107.
Hanson, L.Å. & Wigzell, H.: Immunologi. Teori och Klinik. Almqvist & Wiksell, 5:e upplagan, 1978.
Möller, E.: HLA och Sjukdom. Läkartidningen 1978, 75, 2373-2376.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
