Press release

NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
The Nobel Assembly at the Karolinska Institute has today decided to award the Nobel Prize in Physiology or Medicine for 1992 jointly to
Edmond H. Fischer and Edwin G. Krebs
for their discoveries concerning “reversible protein phosphorylation as a biological regulatory mechanism”.
Summary
Thousands of proteins participate in a complex interplay in a cell. They are the tools of the living organism, regulating its reactions and activities. For example, proteins maintain the metabolic flux, dictate growth and cellular division, release hormones, and mediate muscular work.
Protein interactions are strictly controlled. One of the most important regulatory mechanisms isreversible protein phosphorylation. This means that enzymes phosphorylate and dephosphorylate proteins. Both these enzymatic processes are in turn regulated, often in several steps, allowing amplification and fine control. The 1992 Nobel Prize in Physiology or Medicine is awarded to the American biochemists Edmond Fischer and Edwin Krebs. They purified and characterized the first enzyme of this type. Their fundamental finding initiated a research area which today is one of the most active and wide-ranging.
Reversible protein phosphorylation is responsible for regulation of processes as diverse as mobilization of glucose from glycogen, prevention of transplant rejection by cyclosporin, and development of a cancer form like chronic myeloic leukemia.
Reversible protein phosphorylation
Thousands of proteins participate in the complex interplay in a cell. They constitute the tools of the living organism, regulating all its reactions and activities. For example, proteins maintain the metabolic flux, dictate growth and cellular division, release hormones, and mediate muscular work. Proteins are composed of amino acid residues and have a defined three-dimensional structure. It is this form that dictates the molecular functions. The interactions are strictly regulated. One of the most important mechanisms is phosphorylation of proteins. This means covalent attachment of one or several phosphate groups to the protein (Fig. 1).
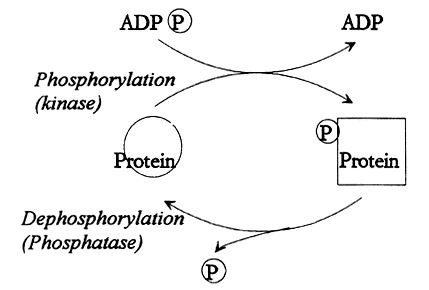 |
Fig. 1. Reversible protein phosphorylation. A protein kinase moves a phosphate group (P) from ATP (ADP(P)) to the protein. The biological properties of the protein is thereby altered. There is also a protein phosphatase that is able to remove the phosphate group. The amount of phosphate that is associated with the protein is thus determined by the relative activities of the kinase and the phosphatase.
The phosphorylation influences the conformation and charge of the protein, thereby also its activity. In this manner, the biological function of a protein can be set at different levels. However, the phosphate groups can also be removed from the protein in a regulated fashion dephosphorylation. This fact constitutes the basis for the designation reversible protein phosphorylation.
The discoveries of Fischer and Krebs
Edmond Fischer and Edwin Krebs characterized the first protein which revealed a novel mechanism for enzyme control through reversible protein phosphorylation. The basic discoveries were made in the mid 1950’s through studies of a special muscle system.
Muscles are composed of a large number of cells capable of contraction or relaxation. For a resting muscle to contract, it has to get energy in the form of sugar, glucose. The glucose is released from glycogen, which is the body storage form of sugar. Glycogen is stored in the liver, and also in muscle cells. When they are told to initiate contractile work, they quickly mobilize their glycogen deposits, converting them to the glucose fuel. In order to achieve this, the organism utilizes a specific glycogen catabolizing protein, termed phosphorylase. This enzyme was discovered by the biochemists Carl and Gerti Cori, bestowing them with the Nobel Prize in Physiology or Medicine in 1947. Enzymes are proteins with the specific role of making biological reactions possible, in short they are catalysts.
It was known that the enzyme phosphorylase can be regulated by small molecules. Edmond Fischer and Edwin Krebs detected that phosphorylase could be converted from an inactive to an active form by a principally novel mechanism. This is carried through by transfer of a phosphate group from the energy-rich compound ATP to the protein. They also showed that this process is catalyzed by an enzyme, a protein kinase.
Enzymes do not only catalyze the attachment of phosphate groups but also their removal. Such enzymes are named phosphatases. In this manner, the glycogen catabolizing phosphorylase is regulated by two enzymes working in opposing directions in a reversible process, one kinase and one phosphatase. Fischer and Krebs, in their fundamental biochemical studies, showed how proteins in the muscle cell rapidly make the energy supply accessible for muscular work.
A mechanism of biological amplification
Step by step, it has become evident that protein phosphorylation constitutes a fundamental mechanism, influencing all cellular functions. For example, Edwin Krebs showed that the effects of cyclic AMP are mediated through a specific protein kinase. Cyclic AMP was discovered by Earl Sutherland (Nobel laureate 1971). It is formed in response to a large number of hormones and molecular signals. The stress hormone, adrenalin (epinephrine), mediates catabolism of glycogen stored in the liver. This liberates glucose into the blood, giving the muscle and heart energy to combat stress.
The fact that cyclic AMP mediates its effects via stimulation of a protein kinase activating the enzyme phosphorylase explains how a hormone signal can lead to quick mobilization of sugar. The serial protein phosphorylations then work as a biological amplifying system (see Fig. 2).
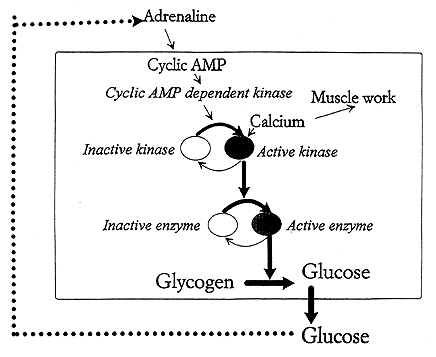
Fig. 2. Protein phosphorylation reactions that are coupled in series can act as a biological amplifier. We are dealing with a controlled chain reaction. When the level of glucose in blood is lowered the amount of the hormone adrenaline rises. This elevates the cyclic AMP content in the liver cell. This activates a cyclic AMP dependent protein kinase, which phosphorylates a kinase that in turn switches on the glycogen degrading enzyme phosphorylase. Hence glycogen is converted to glucose which can enter the blood stream. When the blood glucose rises the adrenaline level in blood goes down. The stimulation is turned off and the phosphatase reactions take over turning the glucose production down. In muscle cells a rise in calcium is the signal for muscular work. Calcium ions also switch on the phosphorylation reactions so that the muscle is provided with the required energy.
Subsequent to these findings of Fischer and Krebs, novel protein kinases are continuously found. We now estimate that perhaps one percent of the genes in the entire genome encode protein kinases. These kinases regulate the function of a large proportion of the thousands of proteins in a cell. In addition, the system includes a large number of phosphatases, which in an opposite manner regulate the removal of the protein phosphate groups from proteins.
Inhibitors and activators
Some of the innumerable cellular processes regulated by reversible protein phosphorylation are shown in Fig. 3. They concern almost all processes important to life. Imbalance between kinases and phosphatases can cause disease and nondesirable tissue reactions. Blood pressure, the inflammatory reaction, and brain signal transduction – just to name a few examples – are being regulated through different hormonal interactions and these interactions in turn are mediated through kinases and phosphatases. We therefore expect the development of drugs which make it possible to influence imbalances by supplying inhibitors and activators directed against the phosphorylation/dephosphorylation components.
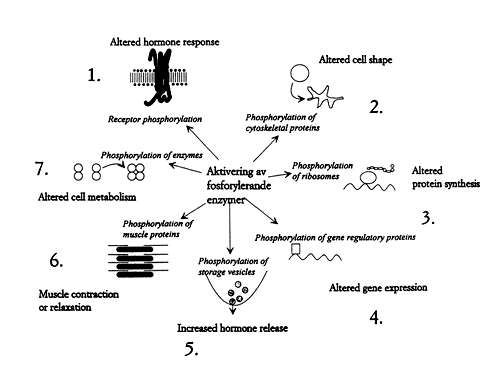 |
Fig. 3. How the cell is affected by protein phosphorylation. 1. Hormone receptors (e.g. the adrenaline receptor) are phosphorylated by specific kinases, which prevent over-stimulation. 2. Phosphorylation can control cell shape and motility. It can even lead to the outgrowth of long processes 3. Phosphorylation of ribosomes affect protein synthesis. 4. Proteins that regulate genes can be reversibly phosphorylated, causing an adapted expression of the genomic information. 5. Hormones and neurotransmitters are contained in storage vesicles. Phosphorylation reactions regulate their release. 6. The proteins that control muscle contraction can be phosphorylated by kinases. Reversible protein phosphorylation thereby affects e.g. blood pressure and respiration. 7. Phosphorylation regulates the enzymes that govern metabolism.
Phosphorylation stimulates cellular growth
The wide-ranging importance of reversible protein phosphorylation makes it difficult to select a single, representative example when so many could be chosen with equal right. However, the activation of the immune response constitutes a suitable model. It illustrates how a series of protein phosphorylations in a cascade amplifies the strength of the initial signal. It further shows how phosphorylation and dephosphorylation intimately interact. The model also gives an insight into work performed by Fischer and Krebs in recent years. The example also shows how drugs that influence phosphorylations are used to save transplants threatened by rejection.
In infections, our immune system is activated by non-self compounds, the antigens. They are consumed by macrophages which transport the antigenic constituents to defined surface structures (Nobel Prize 1980 to Benacerraf, Dausset and Snell). The antigens are then recognized by specialized lymphocytes. The lymphocytes get into contact with the macrophages via a special surface protein. Edmond Fischer showed that this protein works as a phosphatase, removing a phosphate group from an enzyme. This constitutes the start of a chain reaction where a whole cascade of novel phosphorylating enzymes (including several detected by Edwin Krebs) are activated. Their counterparts, the phosphatases, are equally essential in the extended cascade. In the end, an elevated number of specific lymphocytes have been recruited to combat the infection.
However, sometimes the immune defense causes problems, for example following organ transplantations. The recipient’s immune response then attacks the transplanted kidney, liver or pancreas, trying to reject it. Cyclosporin is a drug used with great success in prevention of such graft rejection. It works by intervention of a phosphorylation reaction – it inactivates the phosphatase calcineurin. This enzyme is necessary for development and growth of the specific lymphocytes that attack the transplant.
Under certain conditions, protein phosphorylation can also be of importance for development of cancer. The nuclear DNA of the cell contains a hundred-odd oncogenes. Normally, they produce proteins participating in the regulation of cellular growth. However, should alterations in the oncogenes, mutations, develop, this can lead to formation of products that give abnormal cellular growth, cancer. In several instances an erroneously regulated protein kinase activity is responsible. Chronic myeloic leukemia constitutes such an example.
References
Alberts et al. The Molecular Biology of the Cell. Garland Press, 1990, 2nd edition.
Phosphorylation-dephosphorylation cycle of proteins pp. 129-131, 710-712, 736-737, 777-778.
Fredholm, B. Molekylär farmakologi – vägen till selektiv farmakoterapi. Läkartidningen 1991, 88: 320-421.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
