Popular information
Information for the public:
They opened up a world of possibilities (pdf)
Populärvetenskaplig information:
De öppnade en värld av möjligheter (pdf)

The Nobel Prize in Physiology or Medicine 2012
This year’s Nobel Laureates have shown that life’s path need not be a one-way street. They discovered that the mature cells in our bodies can be turned into stem cells and regain their essentially boundless possibilities.
They opened up a world of possibilities
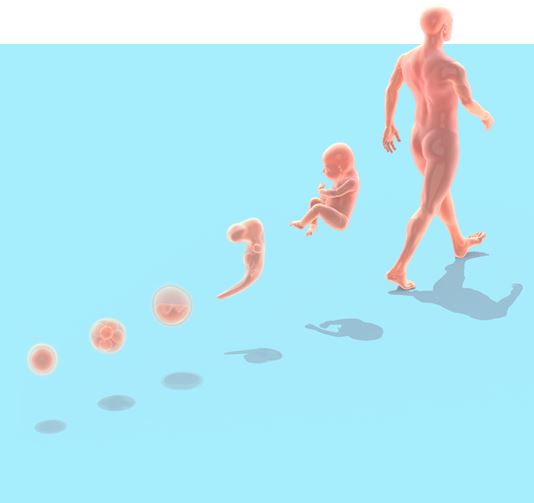
What are you going to be when you grow up? When you are a child, all life’s paths lie open and the question has no answer. But as you move through life a long series of choices and external factors nudge you in a certain direction. When you grow up, maybe you will realise your inner potential by becoming a researcher or a journalist or a politician – but probably not all at the same time.
The cells in our body follow a similar life course. Each individual stem cell in the early embryo could potentially develop into any of the various types of cells that make up the adult body. These versatile cells are called pluripotent stem cells. Soon they start to develop along different pathways to take on specific tasks in the body. Some will become neurons, long thin cells that specialise in sending and receiving nerve impulses; others will turn into the muscle cells that allow us to move, or the bone cells that make up our skeleton.
Your body is full of potential
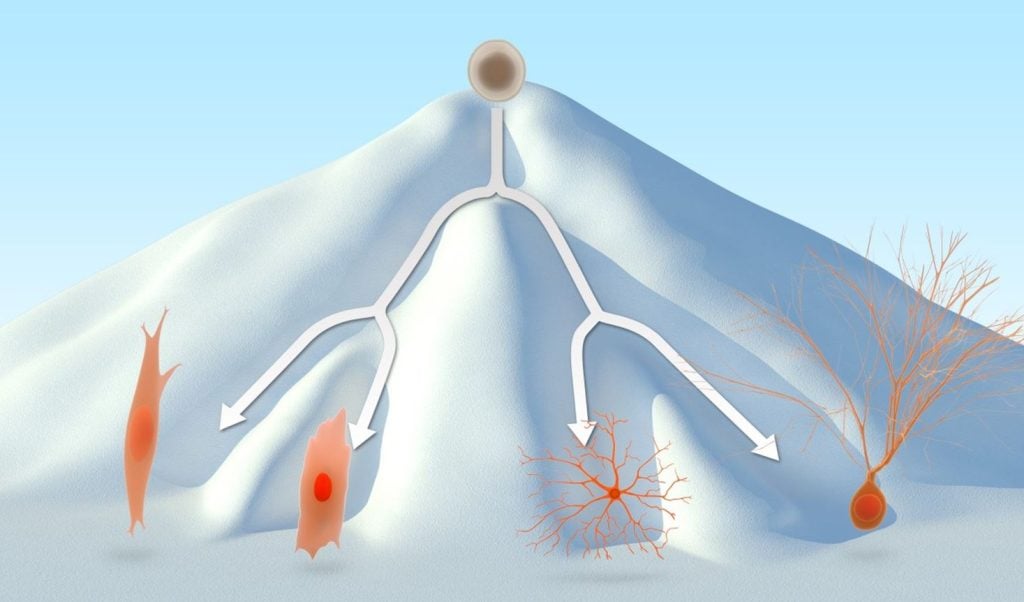
The fertilised egg, the body’s master stem cell, can give rise to all other cell types, a characteristic called totipotency (from Latin totus – “whole”, “entire” and potentia – “ability”, “power”). Totipotency remains through the first few cell divisions (when a complete split of the egg results in identical twins) but is lost soon after.
Pluripotent cells (plures – “several”) can develop into all types of cells except those that form the amniotic sac and the placenta. An early embryo consists mainly of pluripotent stem cells.
Multipotent cells can develop into any of a family of closely related cell types. For example, the blood of adult humans contains multipotent stem cells that can develop into various types of blood cells but not into neurons.
A one-way journey?
For a long time, researchers believed that life’s one-way journey also applied to cells. The doctrine was that once a cell had developed into a specialised cell – a cell with a specific task to do in the body – it had irretrievably lost all the alternative possibilities that had been open to it in the beginning.
There were good reasons for this belief. Granted, a few stem cells remain scattered here and there in the adult body. They serve to generate replacements when specialised cells die, for instance blood cells. Stem cells are able to divide an infinite number of times and can develop into the more specialised cells that need replacing. But no one had ever seen a specialised cell go back to its original form and become a stem cell again. A plausible explanation for this appeared to be that the genetic information in stem cells – the genetic code that gives stem cells their special talents – vanishes or becomes irreversibly suppressed when cells specialise.
A frog-hop backwards in development
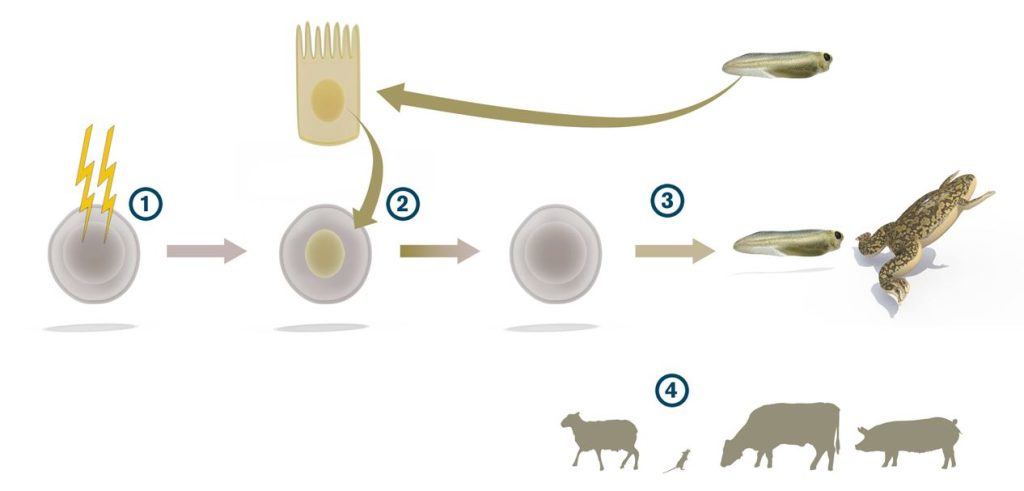
But in 1962 the young embryologist John B. Gurdon presented new research findings that ran counter to the theory of life’s one-way street. Not many believed him at first, but the fact remains: in his laboratory in Oxford, cell nuclei, containing the cell’s genetic material, had jumped backwards in their development.
John B. Gurdon suspected that every cell in the body still contained all the genetic information needed to produce all the body’s various cell types, and he used an ingenious method to test his hypothesis. In his experiments, he first emptied a frog’s egg of its genetic material by destroying the egg cell nucleus with ultraviolet light. Then he replaced the egg’s nucleus with a nucleus from a specialised intestinal cell taken from a tadpole. If the specialised intestinal cell lacked important segments of the genetic material it would not be able to generate tadpoles. But it could – John B. Gurdon’s manipulated frog’s eggs gave rise to both wriggling tadpoles and full-grown frogs.
At first, this new technique called “cloning” did not cause much of a stir in the scientific community. Many researchers doubted the authenticity of the results. But eventually the discoveries were accepted and other researchers began using the technique to study how cells change as they develop and diversify. Several other species of animals were also successfully cloned. In 1997, when British scientists announced the birth of Dolly, the first cloned sheep, it made news worldwide. To date, pigs, cows, wolves and mice have been cloned using various modifications of John B. Gurdon’s technique.
John B. Gurdon demonstrated that the genetic material in the body’s mature cells has the same potential as the genetic material in egg cells. With its help, it is possible to create all the myriad cells of the body – or a whole new individual. In other words, the cells still contained all the genetic information the researchers had previously believed was lost. But the genes are only activated if the nucleus is taken out and transferred into an empty egg cell. The slumbering potential of the adult nucleus is somehow awakened in the egg cell. Theoretically, it should be possible to reprogram the nucleus while it is still in the adult cell, but that would require more detailed knowledge about the intrinsic mechanisms that regulate cell development.
Life there and back again
Shinya Yamanaka was born in 1962, the same year John B. Gurdon reported the arrival of his cloned tadpoles. Forty-four years later, Yamanaka was to find a new recipe to reprogram cells, inspired both by John B. Gurdon’s work and by modern stem cell research. With this recipe in hand, he was the first to induce mature cells to reverse their development and turn back into stem cells.
Early in his career, Shinya Yamanaka dedicated himself to surgery and molecular biology, but he was soon attracted to the many possibilities of pluripotent stem cells. In his laboratory in Nara, Japan, he began to study embryonic stem cells – pluripotent cells that can be harvested from early embryos. He wanted to understand how they retain their pluripotency; in other words, he wanted to figure out what prevents them from developing into specialised cells.
One known characteristic of pluripotent cells was that a specific set of genes are active. These genes encode proteins called transcription factors, which in turn regulate other genes in the cell. When Shinya Yamanaka had succeeded in identifying yet another gene that was important for the pluripotency of embryonic stem cells, he believed he had the knowledge he needed. Now it was time to put it to the test.
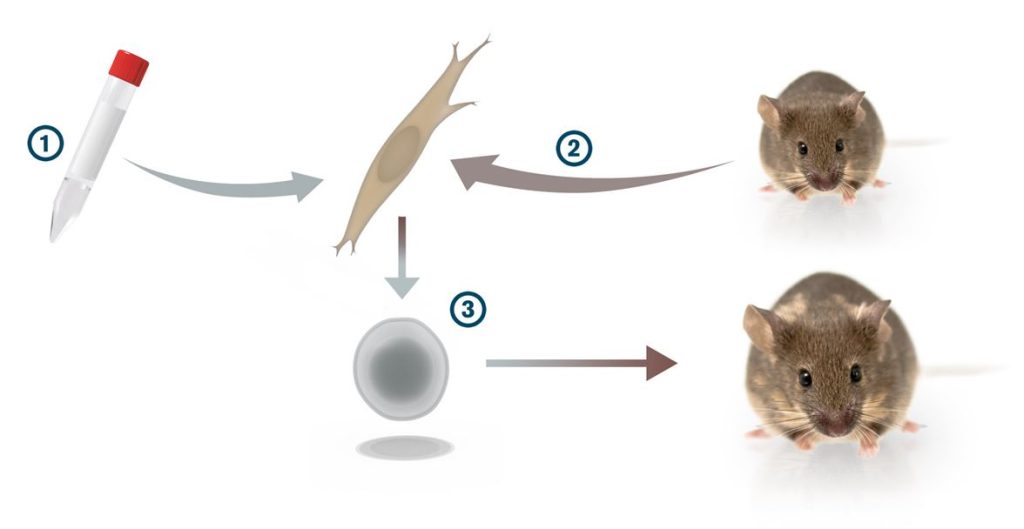
Instead of studying how stem cells are maintained as pluripotent cells, he started at the other end and tried to get specialised cells to turn back into stem cells. After selecting 24 of the genes that were associated with pluripotency, he and his co-workers inserted them into fibroblast cells from the skin of mice, one gene at a time, with the help of a virus. Nothing happened. But when he inserted all 24 genes at once, some of the cells started to change their shape: they plumped up and their nuclei grew. They were converted into something very similar to embryonic stem cells.
It was a groundbreaking discovery, but Shinya Yamanaka wanted to go even farther. He repeated the experiment over and over, gradually reducing the number of genes he inserted. Ultimately he found a surprisingly simple recipe: only four of the genes were required to get the fibroblasts to go into reverse and become pluripotent stem cells.
In later experiments, Shinya Yamanaka was able to demonstrate that the reversed cells – which he called iPS cells (induced pluripotent stem cells) – truly were pluripotent. The iPS cells that he injected into mouse embryos started to diversify into more specialised cells that contributed to the formation of all tissues in the adult mouse.
A technique with many possibilities
When Shinya Yamanaka published his findings in 2006 the scientific community paid very close attention. Before long, researchers all over the world had started experimenting with Yamanaka’s simple basic recipe and soon the technique had developed in several ways.
Shinya Yamanaka had introduced the four genes into the nucleus with the help of retroviruses, which transfer genes to the cell’s genome in a random fashion. This led to inadvertent changes in the cell’s own genes and sometimes gave rise to tumours in the mice. Today researchers have learned other methods of introducing the genes, such as with the help of an RNA molecule, which has no impact on the cell’s own genes. Researchers have also come a long way towards figuring out how to reprogramme specialised cells directly into other types of specialised cells. By inserting just a few transcription factors, it has for example been possible to make skin cells turn directly into heart cells in the laboratory.
One year after Shinya Yamanaka’s breakthrough, he and other researchers succeeded in generating iPS cells from human skin cells. This strengthened the hope that iPS cells might someday be used for medical purposes. In the future scientists hope to be able to use iPS cells to culture cells that can be transplanted into the body and replace diseased cells. Two examples are the dopamine-producing cells in the brain that degenerate in patients with Parkinson disease, and the insulin-producing cells that die off in patients with diabetes. Since iPS cells can be cultured from the patient’s own cells, it should be possible to avoid the immunological reaction that often leads to rejection of cells transplanted from another individual. This research is still at an early stage and many problems remain to be solved before transplantation of iPS cells becomes a clinical option. For instance, two of the four genes on the basic recipe’s list of ingredients are known to be active in tumours. The technique must be evaluated in view of the risk that iPS cells transplanted into a patient’s body might start to replicate out of control and give rise to cancer.
The iPS technique also means that researchers now have an unlimited source for stem cells. Stem cell research has relied heavily of embryonic stem cells harvested from embryos. Human embryos are not easy to come by, and many consider research involving use of human embryonic stem cells to be ethically questionable. Embryonic stem cells will continue to be of great scientific interest, but in many situations researchers can now use iPS cells instead.
Diseases in a dish

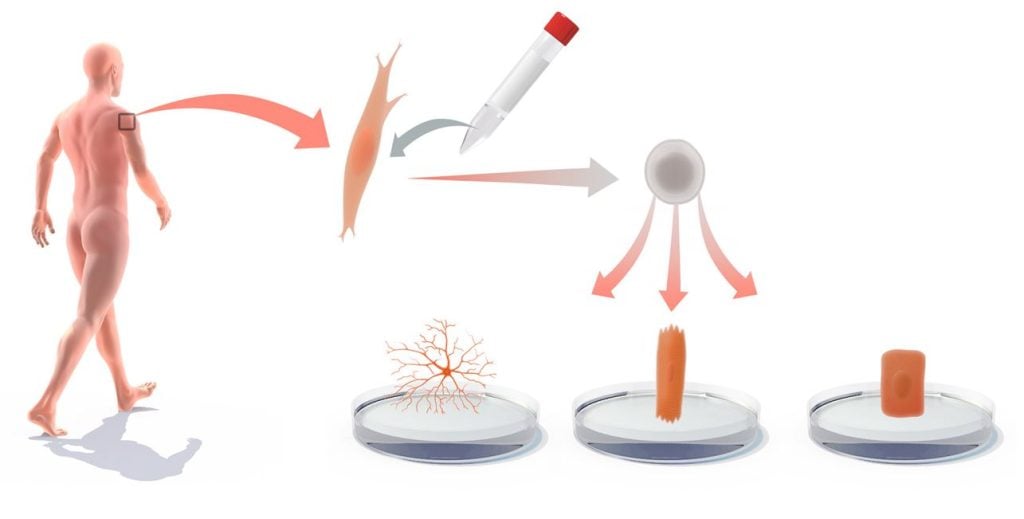
Another application – one that has already become reality – is to use iPS cells to learn more about diseases. Many diseases arise when certain types of cells in the body are not working the way they should. But the exact causes are often difficult to study because the cells are tucked away in inaccessible parts of the body, such as the brain or the heart.
Using iPS technique, researchers can grow copies of the body’s diseased cells. All that is required is a skin sample from the patient and Shinya Yamanaka’s recipe for reprogramming. The cells from the patient’s skin are first converted into iPS cells, and then they are induced to differentiate into the type of cell the researchers want to study. These cultured cells have exactly the same genes and mutations as the cells in the patient’s body. This makes the method particularly suitable for studying hereditary diseases. Researchers currently use this method to examine how cells function in a whole series of disease states: they study the nerve cells that activate muscles and that atrophy in patients with amyotrophic lateral sclerosis (Lou Gehrig’s disease) and the brain cells that die in patients with Alzheimer’s disease. In the same way, this technique can be used to study what effects various pharmaceuticals have on the diseased cells.
The journey has just begun
Gurdon’s and Yamanaka’s discoveries have shown that specialised cells can, under certain conditions, go into reverse. The changes that occur in a cell’s genes as it moves along the normal path of development are not irrevocable. We have a new perspective on how cells and organisms develop.
John B. Gurdon, with his manipulated frog’s eggs, and Shinya Yamanaka, with his iPS cells, have demonstrated that every cell in our body bears within it the potential to convert into stem cells. But to explore the possible medical applications of this insight, we must look to the road ahead. The Nobel Laureates’ discoveries opened a new door and the world’s scientists have set forth on a long journey of exploration, hoping to find our cells’ true potential.
The Laureates
SIR JOHN B. GURDON (1933– )
Group leader at the Gurdon Institute in Cambridge. He has also served as Professor of Zoology at Cambridge University and as Master of Magdalene College in Cambridge.
SHINYA YAMANAKA (1962– )
Professor at Kyoto University and Director of the Center for iPS Cell Research and Application. He is also a senior investigator at the Gladstone Institutes in San Francisco.
The editorial committee for this year’s popular presentation of the Nobel Prize in Physiology or Medicine included the following scientific advisors, all professors at Karolinska Institutet: Göran K Hansson, Medicine, Secretary of the Nobel Assembly; Urban Lendahl, Genetics, Chairman of the Nobel Committee; Jonas Frisén, Stem cell research; Thomas Perlmann, Molecular developmental biology.
Text: Ola Danielsson, medical journalist
Translation: Janet Holmén, editor
Illustrations and layout: Mattias Karlén
© The Nobel Committee for Physiology or Medicine, Karolinska Institutet
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
