Press release

12 October 1994
The Royal Swedish Academy of Sciences has decided to award the 1994 Nobel Prize in Physics for pioneering contributions to the development of neutron scattering techniques for studies of condensed matter with one half to Professor Bertram N. Brockhouse, McMaster University, Hamilton, Ontario, Canada, for the development of neutron spectroscopy
and one half to Professor Clifford G. Shull, MIT, Cambridge, Massachusetts, USA for the development of the neutron diffraction technique
The structure and dynamics of matter revealed
Most people know that X-ray methods and microscopy can be used for studying objects in detail. Despite refinements these methods are not always adequate. The researchers now rewarded have developed neutron scattering techniques, powerful methods of analysing both solid and fluid (condensed) matter. The techniques were developed at the relatively simple and not-too-powerful nuclear reactors that became available to researchers shortly after the second World War. Successive developments have led to today’s large installations specially built for studies of condensed matter in, for example, France, England and the USA, and more are planned.
Both methods are based on the use of neutrons flowing out from a nuclear reactor. When the neutrons bounce against (are scattered by) atoms in the sample being investigated, their directions change, depending on the atoms’ relative positions. This shows how the atoms are arranged in relation to each other, that is, the structure of the sample. Changes in the neutrons’ velocity, however, give information on the atoms’ movements, e.g. their individual and collective oscillations, that is their dynamics. In simple terms, Clifford G. Shull has helped answer the question of where atoms “are” and Bertram N. Brockhouse the question of what atoms “do”.
Neutron scattering techniques are used in such widely differing areas as the study of the new ceramic superconductors, catalytic exhaust cleaning, elastic properties of polymers and virus structure.
Dynamic development
Brockhouse and Shull made their pioneering contributions at the first nuclear reactors in the USA and Canada as early as the 1940s and l950s. Neutron scattering techniques have since developed considerably and in the past few years neutrons have been used to an increasing extent for studying the structure (arrangement) and dynamics (movement) of solid and fluid matter. The number of researchers is now reckoned in thousands, with intensive research at the many neutron scattering installations the world over. The high flux reactor at the Institut Laue-Langevin at Grenoble, France, is an example of a large European research plant from the beginning of the 1970s (recently upgraded). Studies here include both the structure and the dynamics of the new ceramic superconductors (Nobel Prize 1987 to Bednorz and Müller), molecule movements on surfaces of relevance to catalytic exhaust cleaning, the structure of viruses and how these defend themselves against dehydration, and the connection between the ordered and the non-ordered structures of polymers and their elastic properties (Nobel Prize 1991 to de Gennes). The handbook for researchers wishing to use the installation describes no fewer than 16 instruments for studying structure and 14 for dynamics.
At the Rutherford Appleton Laboratory in England an accelerator-based neutron source (ISIS) was built for similar purposes and at NIST (the National Institute of Science and Technology) in the USA there is a 1990 variant of the Grenoble installation. It is now planned to open new and very advanced installations in Europe, the USA and Asia. Using these it is hoped to gain new basic knowledge, but also to develop technological applications (computer memory) and environmental applications (the chemistry of pollution).
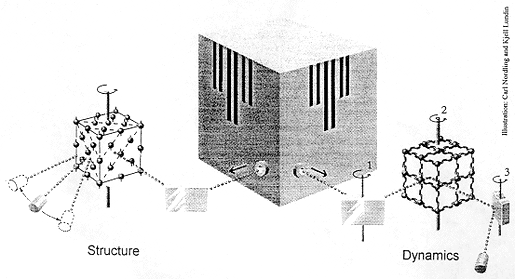
What happens
The illustration shows how neutrons from a research reactor may be used for
studying structure and dynamics.
In the left-hand part of the picture the neutron beam is first reflected in a crystal. Because of the wave nature of the neutrons – a characteristic of all moving particles -and the strict arrangement of the crystal atoms in a regular pattern, the neutrons reflected in a certain direction will have a defined wavelength (the Bragg condition). With the crystal set at a suitable angle, a certain neutron wavelength can be selected. These “monochromatised” neutrons then irradiate the sample to be investigated. Since neutrons are electrically neutral, they have great penetrability and hence search the whole sample. Most of the neutrons leave the sample with unchanged energy (elastic scattering) and a preference for certain directions (diffraction). By counting the neutrons in a rotatable detector, a diffraction pattern is obtained which shows the relative positions of the atoms in the sample. It is for the development of this variant of the neutron scattering technique that Clifford G. Shull has been awarded his Nobel Prize. He has shown how neutrons may be used to determine the atomic structure of a material.
The right-hand part of the picture shows the basic principle used by Brockhouse. Neutrons from the reactor are first monochromatised by a crystal which may be turned about an axis (1). When the neutrons penetrate the sample, which is rotatable about another axis (2), they can initiate or cancel out oscillations in the sample’s atoms. These movements, in which all atoms participate collectively, are called phonons. If the neutrons manage to create (excite) phonons, they themselves lose energy (inelastic scattering). When the neutrons have left the sample their energy is analysed in a crystal which can be turned about a third axis (3) and finally counted in a detector. Using an apparatus of this type – a triple-axis spectrometer – movements, the dynamics, of a material or a crystal may be studied. It is for the development of this technique, neutron spectroscopy for condensed matter, that Bertram N. Brockhouse has been awarded his Nobel Prize.
How it all began
At the end of the second World War researchers in the USA gained access to the large neutron fluxes that even relatively modest nuclear reactors were capable of delivering. Neutrons had then been known as building blocks in the atomic nucleus for more than a decade (Nobel Prize to Chadwick in 1935 for their discovery). Enrico Fermi showed in 1942 that neutrons from fission of the uranium nucleus could support a controlled chain reaction. He had earlier made the important discovery that slowed-down or thermal neutrons show a much greater inclination to react than fast ones do (Nobel Prize for this discovery, among others, to Fermi in 1938). It is the special properties of these slow neutrons that make them suitable for detecting the positions and movements of atoms. Even before the entry of the nuclear reactors into the research arena, results of using simple neutron sources had indicated that neutron beams could be used for studying solid bodies and liquids. However, there were many difficulties to be overcome before these possibilities could be realised.
At the nuclear reactor at Oak Ridge in the USA the late E.O.Wollan formed a working group to examine the possibilities of developing neutron beams and apparatuses for determining structure. Clifford Shull was early linked to this group and was soon to play a major part. Similar efforts were being made elsewhere but it was the Wollan-Shull group and later Shull in collaboration with other researchers, that proceeded most purposively and achieved results with surprising rapidity. Shull’s studies of simple crystals laid a basis for the interpretation of the very complicated structures being analysed by modern neutron crystallographers.
Where are the hydrogen atoms?
Hydrogen is one of the commonest elements in biological matter. It also occurs in many forms of technically important inorganic matter. The localisation of hydrogen in the structures of these would have been practically impossible with the earlier X-ray diffraction method (for which von Laue and the Braggs, father and son, became Nobel laureates in 1914-1915) since the hydrogen atom gives a scarcely noticeable scattering of X-ray radiation. (X-ray beams are scattered against electrons in the diffracting atoms, and the hydrogen atom has only one electron). As opposed to this, the nucleus of the hydrogen atom, the proton, constitutes a very efficient neutronscattering centre and its position can therefore be determined with neutron diffraction. Through his first successful experiments Shull opened what was to become a very large field for finding out how hydrogen is bound in, for example, ice, metallic hydrides and organic compounds.
Magnetic structures
Neutrons are small magnets, as are the atoms of a magnetic material. When a neutron beam strikes such material, the neutrons can therefore change direction through magnetic interaction with the atoms of the material. This gives rise to a new type of neutron diffraction (the type described earlier is based on neutron interaction with atomic nuclei) which can be used to study the relative orientations of the small atomic magnets. Here, too, the X-ray method has been powerless and in this field of application neutron diffraction has since assumed an entirely dominant position. It is hard to imagine modern research into magnetism without this aid.
A new breakthrough
While Shull was developing the neutron scattering technique based on the diffraction of elastically scattered neutrons, Brockhouse at the Chalk River research reactor, in Canada, was concentrating chiefly on inelastic scattering. He designed the triple-axis spectrometer already mentioned and developed methodology for studying the energy spectrum of the neutrons once they had been scattered. This required profound knowledge of the properties of neutrons and great ingenuity. It was only with Brockhouse’s contributions that inelastic neutron scattering became a useful tool in the physics of condensed matter. Neutrons again proved to have unique scattering properties, in this case because their energy is of the same order of magnitude as that of the phonons in solid and fluid matter. During a hectic period between 1955 and 1960 Brockhouse’s pioneering work was without parallel within neutron spectroscopy. This enabled the technique to develop into an in many ways unique source of information which has revolutionised our ability to chart atomic dynamics, e.g. atomic vibrations in crystals, diffusion movements in liquids and fluctuations in magnetic material. Such information is contributing actively to the elucidation of the forces that bind atoms to one another in solid bodies and that determine, for instance, the transition from the solid state to the fluid state.
Phonons and magnons
The number of atoms in a macroscopic quantity of matter is very large, giving rise to a rich flora of movement types in solid and fluid bodies. The connection between energy and wavelength in, for example, crystal oscillations, termed the phonon dispersion relation, is a complicated function. The shape of the dispersion curve is, however, characteristic for a crystal, and mapping this affords valuable information on the properties of materials. As early as in 1955 Brockhouse and A.T. Stewart reported results concerning phonons in, among other things, aluminium crystals, and they specified for the first time an experimentally measured dispersion curve for these.
In crystals of magnetic material, e.g. magnetite, a type of collective wave motion can occur among the atomic elementary magnets. This wave motion can be excited by neutrons, and Brockhouse was the first to study and measure the dispersion curve for the elementary excitations of this wave motion, termed magnons.
Non-ordered movement
For non-ordered movement in fluids and melts, as in magnets, the late L. Van Hove formulated, in the early 1950s, a theory of how the memory of a certain arrangement of atoms, gradually disappears over time. Neutrons make it possible to follow the change of atomic structures over time. Brockhouse was the first to show experimentally how these ‘correlation’ or ‘memory’ functions could be determined using neutron scattering in experiments with, among other substances, H2O (water) and D2O (heavy water). In the same way, his experiments with liquid lead provided a model for others to follow.
Such experiments provided the starting point for a lively development of theories for liquids and non-ordered systems in general. Phenomena such as lattice dynamics and diffusion underwent a renaissance through these and subsequent research contributions.
Through the studies of atomic structure and dynamics made possible by Bertram N. Brockhouse and Clifford G. Shull with their development of neutron scattering techniques, valuable information is being obtained for use in e.g. the development of new materials. An important example is the ceramic superconductors now being studied intensively, although these have not yet been developed for commercial use.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
