Popular information
English
Swedish

The Nobel Prize in Chemistry 2000
We are used to the great impact scientific discoveries have on our ways of thinking. This year’s Nobel Prize in Chemistry is no exception. What we have been taught about plastic is that it is a good insulator – otherwise we should not use it as insulation in electric wires. But now the time has come when we have to change our views. Plastic can indeed, under certain circumstances, be made to behave very like a metal – a discovery for which Alan J. Heeger, Alan G. MacDiarmid and Hideki Shirakawa are to receive the Nobel Prize in Chemistry 2000.
How can plastic become conductive?
Plastics are polymers, molecules that form long chains, repeating themselves like pearls in a necklace. In becoming electrically conductive, a polymer has to imitate a metal, that is, its electrons need to be free to move and not bound to the atoms. The first condition for this is that the polymer consists of alternating single and double bonds, called conjugated double bonds. Polyacetylene, prepared through polymerization of the hydrocarbon acetylene, has such a structure:
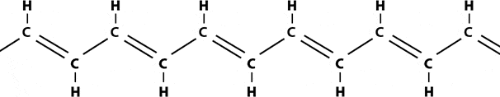 |
| Polyacetylene |
However, it is not enough to have conjugated double bonds. To become electrically conductive, the plastic has to be disturbed – either by removing electrons from (oxidation), or inserting them into (reduction), the material. The process is known as doping.
The game in the illustration to the right offers a simple model of a doped polymer. The pieces cannot move unless there is at least one empty “hole”. In the polymer each piece is an electron that jumps to a hole vacated by another one. This creates a movement along the molecule – an electric current.
This model is greatly over-simplified, and we shall consider a more “chemical” model later.
High resolution(JPEG 155 kb)
What Heeger, MacDiarmid and Shirakawa found was that a thin film of polyacetylene could be oxidised with iodine vapour, increasing its electrical conductivity a billion times. This sensational finding was the result of their impressive work, but also of coincidences and accidental circumstances. Let us, shortly, tell the story of one of the great chemical discoveries of our time.
How polymer conductivity was revealed – and the importance of a coffee-break
The leading actor in this story is the hydrocarbon polyacetylene, a flat molecule with an angle of 120° between the bonds and hence existing in two different forms, the isomers cis-polyacetylene and trans-polyacetylene (the latter form illustrated above). At the beginning of the 1970s, the Japanese chemist Shirakawa found that it was possible to synthetisize polyacetylene in a new way, in which he could control the proportions of cis– and trans-isomers in the black polyacetylene film that appeared on the inside of the reaction vessel. Once – by mistake – a thousand-fold too much catalyst was added. To Shirakawa’s surprise, this time a beautiful silvery film appeared.
Shirakawa was stimulated by this discovery. The silvery film was trans-polyacetylene, and the corresponding reaction at another temperature gave a copper-coloured film instead. The latter film appeared to consist of almost pure cis-polyacetylene. This way of varying temperature and concentration of catalyst was to become decisive for the development ahead.
In another part of the world, chemist MacDiarmid and physicist Heeger were experimenting with a metallic-looking film of the inorganic polymer sulphur nitride, (SN)x. MacDiarmid referred to this at a seminar in Tokyo. Here the story could have come to a sudden end, had not Shirakawa and MacDiarmid happened to meet, accidentally, during a coffee-break.
When MacDiarmid heard about Shirakawa’s discovery of an organic polymer that also gleamed like silver, he invited Shirakawa to the University of Pennsylvania in Philadelphia. They set about modifying polyacetylene by oxidation with iodine vapour. Shirakawa knew that the optical properties changed in the oxidation process and MacDiarmid suggested that they ask Heeger to have a look at the films. One of Heeger’s students measured the conductivity of the iodine-doped trans-polyacetylene and – eureka! The conductivity had increased ten million times!
In the summer of 1977, Heeger, MacDiarmid, Shirakawa, and co-workers, published their discovery in the article “Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene (CH)n” in The Journal of Chemical Society, Chemical Communications. The discovery was considered a major breakthrough. Since then the field has grown immensely, and also given rise to many new and exciting applications. We shall return to some of them.
Doping – for better molecule performance
What exactly happened in the polyacetylene films? When we compare some common compounds with regard to conductivity, we see that the conductivities of the polymers vary considerably. Doped polyacetylene is, e.g., comparable to good conductors such as copper and silver, whereas in its original form it is a semiconductor.
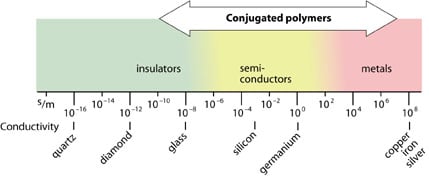 |
A metal wire conducts electric current because the electrons in the metal are free to move. How then do we explain the conductivity of the doped polymers?
When describing polymer molecules we distinguish between ![]() (sigma) bonds and
(sigma) bonds and ![]() (pi) bonds. The
(pi) bonds. The ![]() bonds are fixed and immobile. They form the covalent bonds between the carbon atoms. The
bonds are fixed and immobile. They form the covalent bonds between the carbon atoms. The ![]() electrons in a conjugated double bond system are also relatively localised, though not as strongly bound as the
electrons in a conjugated double bond system are also relatively localised, though not as strongly bound as the ![]() electrons. Before a current can flow along the molecule one or more electrons have to be removed or inserted. If an electrical field is then applied, the electrons constituting the
electrons. Before a current can flow along the molecule one or more electrons have to be removed or inserted. If an electrical field is then applied, the electrons constituting the ![]() bonds can move rapidly along the molecule chain. The conductivity of the plastic material, which consists of many polymer chains, will be limited by the fact that the electrons have to “jump” from one molecule to the next. Hence, the chains have to be well packed in ordered rows.
bonds can move rapidly along the molecule chain. The conductivity of the plastic material, which consists of many polymer chains, will be limited by the fact that the electrons have to “jump” from one molecule to the next. Hence, the chains have to be well packed in ordered rows.
As mentioned earlier, there are two types of doping, oxidation or reduction. In the case of polyacetylene the reactions are written like this:
Oxidation with halogen (p-doping): [CH]n + 3x/2 I2 –> [CH]nx+ + x I3–
Reduction with alkali metal (n-doping): [CH]n + x Na –> [CH]nx- + x Na+
The doped polymer is a salt. However, it is not the iodide or sodium ions that move to create the current, but the electrons from the conjugated double bonds. Furthermore, if a strong enough electrical field is applied, the iodide and sodium ions can move either towards or away from the polymer. This means that the direction of the doping reaction can be controlled and the conductive polymer can easily be switched on or off.
Polarons – doped carbon chains
In the first of the above reactions, oxidation, the iodine molecule attracts an electron from the polyacetylene chain and becomes I3– . The polyacetylene molecule, now positively charged, is termed a radical cation, or polaron (fig. b below).
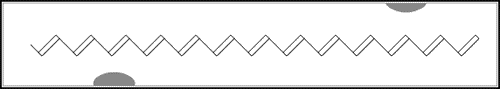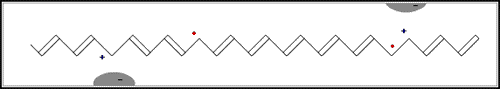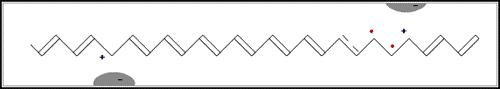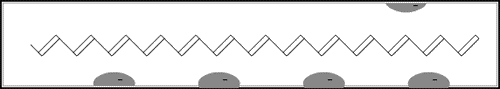
The lonely electron of the double bond, from which an electron was removed, can move easily. As a consequence, the double bond successively moves along the molecule. The positive charge, on the other hand, is fixed by electrostatic attraction to the iodide ion, which does not move so readily. If the polyacetylene chain is heavily oxidised, polarons condense pair-wise into so-called solitons. These solitons are then responsible, in complicated ways, for the transport of charges along the polymer chains, as well as from chain to chain on a macroscopic scale.
We have only touched upon the complex theory that explains how polymers can be made electrically conductive. We recommend the longer, and more detailed, version “Information (advanced) on the Nobel Prize 2000” for everybody who feels challenged to go deeper into the subject.
Brilliant applications
Metal wires that conduct electricity can be made to light up when a strong enough current is passing – as we are reminded of every time we switch on a light bulb. Polymers can also be made to light up, but by another principle, namely electroluminescence, which is used in photodiodes. These photodiodes are, in principal, more energy saving and generate less heat than light bulbs.
In electroluminescence, light is emitted from a thin layer of the polymer when excited by an electrical field. In photodiodes inorganic semiconductors such as gallium phosphide are traditionally used, but now one can also use semiconductive polymers.
Electroluminescence from semiconductive polymers has been known for about ten years. Today there is extensive commercial interest in photodiodes and in light-emitting diodes (LEDs). A LED can consist of a conductive polymer as an electrode on one side, then a semiconductive polymer in the middle and, at the other end, a thin metal foil as electrode. When a voltage is applied between the electrodes, the semiconductive polymer will start emitting light.
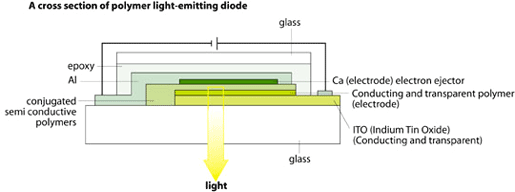 |
| High resolution (JPEG 174 kb) |
There are many applications of this brilliant plastic. In a few years, for example, flat television screens based on LED film will become reality, as will luminous traffic signs and information signs. Since it is relatively simple to produce large, thin layers of plastic, one can also imagine light-emitting wallpaper in our homes, and other spectacular things.
More applications
Some applications of conductive polymers that have come onto the market, or are undergoing trials, are:
- Polythiophene derivates, that are of great commercial use in antistatic treatment of photographic film. They can also be used in devices in supermarkets for marking products. The checkouts will then automatically register what the customer has in the trolley.
- Doped polyaniline in antistatic material, e.g. in plastic carpets for offices and operating theatres, where it is important to avoid static electricity. It is also used on computer screens, protecting the user from electromagnetic radiation, and as a corrosion inhibitor.
- Materials such as polyphenylenevinylene may soon be used in mobile phone displays.
- Polydialkylfluorenes are used in the development of new colour screens for video and TV.
With plastic into the future
In the 20th century we had telephones of Bakelite, stockings of nylon, bags of polythene and thousands of other more or less essential plastic objects. What does our new century offer? Perhaps we will use plastics differently now, in the light of this year’s Nobel Prize in Chemistry.
One reason for the great commercial potential of conductive and semiconductive polymers is that they can be produced quickly and cheaply. Electronic components based on polymers, and polymer-based integrated circuits, will soon find their place in consumer products where low processing costs will be more important than high speed.
The step from polymer-based electronics to real molecular-scale electronics is a large but fascinating one. Molecule-based integrated circuits could be reduced to a scale many orders of magnitudes smaller than silicon-based electronics allows. While many challenges lie ahead, we stand at the threshold to a plastic-electronics revolution with exciting implications in chemistry and physics as well as information technology.
The Laureates
Alan J. Heeger (born 1936) received his Ph.D. at University of California, Berkeley 1961 and became associate professor at University of Pennsylvania 1962 and had a professorship there between 1967 and 1982. Since 1982 he is a Professor of Physics at University of California at Santa Barbara and director for the Institute for Polymers and Organic Solids. In 1990 he founded UNIAX Corporation where he is Chair of the Board.
Prof. Alan J. Heeger
Institute for Polymers and Organic Solids
And Department of Physics and Materials
University of California at Santa Barbara
Santa Barbara, CA 93106-5090
Alan G. MacDiarmid (born 1927) grew up in New Zealand, and received his Ph.D. at University of Wisconsin 1953 and at University of Cambridge, UK, 1955. He was associate professor at University of Pennsylvania 1956 and received a professorship there 1964. Since 1988 he is Blanchard Professor of Chemistry.
Prof. Alan G. MacDiarmid
University of Pennsylvania
34th and Spruce Streets
Philadelphia, PA 19104
Hideki Shirakawa (born 1936) received his Ph.D. at Tokyo Institute of Technology 1966 became associate professor at the Institute of Materials Science at University of Tsukuba 1966. He is a Professor there since 1982.
Prof. Hideki Shirakawa
Institute of Materials Sciences
University of Tsukuba
Sakura-mura, Ibaraki 305, Japan
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
