Popular information
Popular science background:
How molecules became machines (pdf)
Populärvetenskaplig information:
Hur molekyler blev till maskiner (pdf)

The Nobel Prize in Chemistry 2016 is awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart and Bernard L. Feringa for their development of molecular machines that are a thousand times thinner than a hair strand. This is the story of how they succeeded in linking molecules together to design everything from a tiny lift to motors and minuscule muscles.
How molecules became machines
How small can you make machinery? This is the question that Nobel Laureate Richard Feynman, famed for his 1950s’ predictions of developments in nanotechnology, posed at the start of a visionary lecture in 1984. Barefoot, and wearing a pink polo top and beige shorts, he turned to the audience and said: “Now let us talk about the possibility of making machines with movable parts, which are very tiny.”
He was convinced it was possible to build machines with dimensions on the nanometre scale. These already existed in nature. He gave bacterial flagella as an example, corkscrew-shaped macromolecules which, when they spin, make bacteria move forward. But could humans – with their gigantic hands – build machines so small that you would need an electron microscope to see them?
A vision of the future – molecular machines will exist within 25–30 years
One possible way would be to build a pair of mechanical hands that are smaller than your own, which in turn build a pair of smaller hands, which build even smaller hands, and so on, until a pair of minuscule hands can build equally minuscule machinery. This has been tried, said Feynman, but without great success.
Another strategy, in which Richard Feynman had more faith, would be to build the machinery from the bottom up. In his theoretical construction, different substances, such as silicon, are sprayed onto a surface, one layer of atoms after another. Afterwards, some layers are partially dissolved and removed, creating moving parts that can be controlled using an electric current. In Feynman’s vision of the future, such a construction could be used to create an optical shutter for a tiny camera.
The aim of the lecture was to inspire the researchers in the audience, to get them to test the limits of what they believed possible. When Feynman finally folded up his notes, he looked out at the audience and said, mischievously: “… have a delightful time in redesigning all kinds of familiar machinery, to see if you can do it. And give it 25–30 years, there will be some practical use for this. What it is, I do not know.”
What neither Feynman, nor the researchers in the audience, knew at the time was that the first step towards molecular machinery had already been taken, but in a rather different way to that predicted by Feynman.
Mechanically interlocked molecules
In the mid-20th century, as part of efforts to build increasingly advanced molecules, chemists were attempting to produce molecular chains in which ring-shaped molecules were linked together. The person who succeeded would not just create an amazing new molecule, but also a new type of bond. Normally, molecules are held together by strong covalent bonds in which atoms share electrons. The dream was to instead create mechanical bonds, where molecules are interlocked without the atoms interacting directly with each other (figure 1).
In the 1950s and 1960s, several research groups reported that their test tubes contained molecular chains, but the amounts they produced were small and the methods so complex that they were of limited use. Progress was regarded more as a curiosity than as functional chemistry. After years of setbacks, many people gave up hope and, in the beginning of the 1980s, the field was beset by weariness. However, the major breakthrough came in 1983. Using an ordinary copper ion, a French research group, led by chemist Jean-Pierre Sauvage, took control of the molecules.
Jean-Pierre Sauvage gathers molecules around a copper ion
As so often happens in research, inspiration arrived from a completely different field. Jean-Pierre Sauvage worked with photochemistry, in which chemists develop molecular complexes that can capture the energy contained in the sun’s rays and utilise it to drive chemical reactions. When Jean- Pierre Sauvage built a model of one of these photochemically active complexes, he suddenly saw its similarity to a molecular chain: two molecules were intertwined around a central copper ion.
This insight led to a dramatic turn in the direction of Jean-Pierre Sauvage’s research. Using the photochemical complex as a model, his research group constructed one ring-shaped and one crescent-shaped molecule so that they were attracted to a copper ion (figure 1); the copper ion provided a kind of cohesive force that held the molecules together. In a second step, the group used chemistry to weld together the crescent-shaped molecule with a third molecule so a new ring was formed, thereby creating the first link in a chain. The researchers could then remove the copper ion, which had served its purpose.
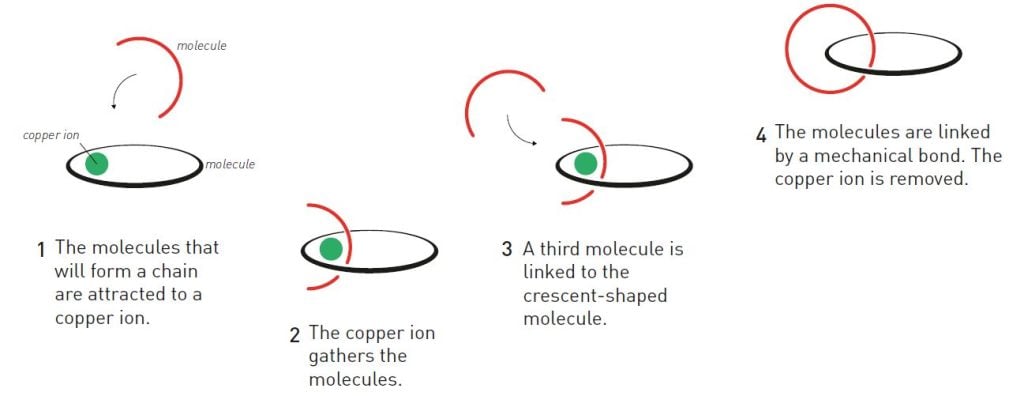
Chemists talk about the yield of a reaction: the percentage of the initial molecules that form the target molecule. In previous attempts to create linked molecules, researchers had at best achieved a yield of a few per cent. Thanks to the copper ion, Sauvage was able to increase the yield to an impressive 42 per cent. Suddenly, molecular chains were more than just a curiosity.
With the help of this revolutionary method, Sauvage reinvigorated the field of topological chemistry, in which researchers – often using metal ions – interlock molecules in increasingly complex structures, from long chains to complicated knots. Jean-Pierre Sauvage and J. Fraser Stoddart (we will return to him soon) are leaders in this field and their research groups have created molecular versions of cultural symbols such as the trefoil knot, Solomon’s knot and the Borromean rings (figure 2).
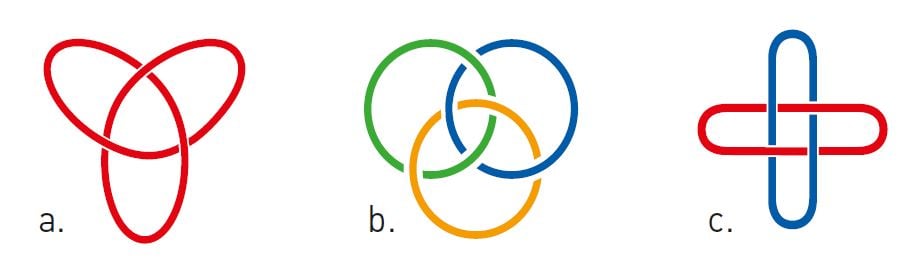
However, aesthetic molecular knots are a diversion in the story of 2016’s Nobel Prize in Chemistry – back to molecular machinery.
… and takes the first step towards a molecular motor
Jean-Pierre Sauvage soon realised that molecular chains (called catenanes, from the Latin word for chain, catena) were not only a new class of molecule, but that he had also taken the first step towards creating a molecular machine. In order for a machine to perform a task, it must consist of several parts that can move in relation to each other. The two interlocking rings fulfilled this requirement. In 1994, Jean-Pierre Sauvage’s research group also succeeded in producing a catenane in which one ring rotated, in a controlled manner, one revolution around the other ring when energy was added. This was the first embryo of a non-biological molecular machine.
The second embryo of a molecular machine was produced by a chemist who grew up on a farm without electricity or any modern-day conveniences in Scotland.
Fraser Stoddart threads a molecular ring onto a molecular axle
As a child, J. Fraser Stoddart had no television or computer. Instead, to occupy himself he did jigsaws, so training a skill that chemists need: recognising shapes and seeing how they can be linked together. He was also attracted to chemistry by the prospect of becoming a molecular artist – sculpting new shapes, ones the world had never seen before.
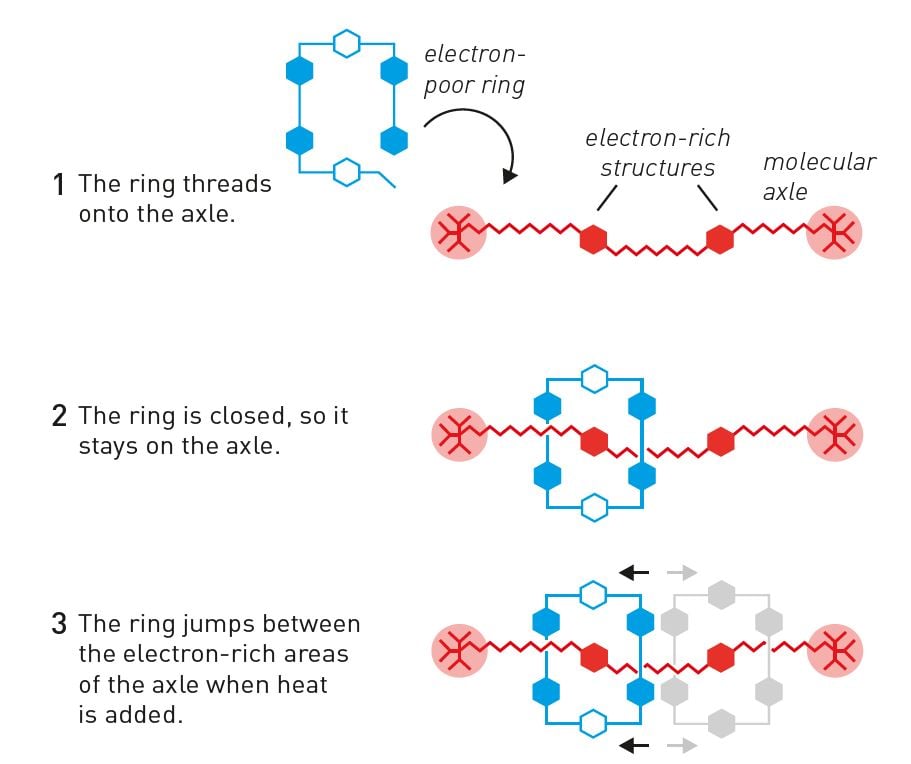
When Fraser Stoddart developed one of the molecular creations that is the foundation of 2016’s Nobel Prize in Chemistry, he also utilised chemistry’s potential for designing molecules that are attracted to each other. In 1991, his research group built an open ring that lacked electrons, and a long rod, or axle, that had electron-rich structures in two places (figure 3). When the two molecules met in a solution, electron-poor was attracted to electron-rich, and the ring threaded onto the axle. In the next step, the research group closed the opening in the ring so that it remained on the molecular axle. He had thus, with a high yield, created a rotaxane: a ring-shaped molecule that is mechanically attached to an axle.
Fraser Stoddart then made use of the ring’s freedom to move along the axle. When he added heat the ring jumped forwards and backwards – like a tiny shuttle – between the two electron-rich parts of the axle (figure 3). In 1994, he could completely control this movement, thereby breaking away from the randomness that otherwise governs movements in chemical systems.
A lift, a muscle and a minuscule computer chip
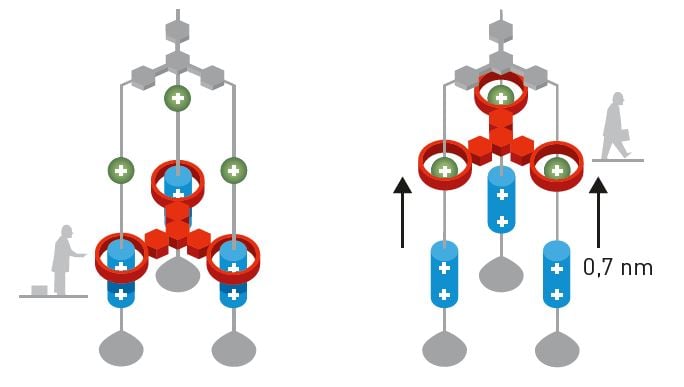
Since 1994, Stoddart’s research group has used various rotaxanes to construct numerous molecular machines, including a lift (2004, figure 4), which can raise itself 0.7 nanometres above a surface, and an artificial muscle (2005), where rotaxanes bend a very thin gold lamina.
In partnership with other researchers, Fraser Stoddart has also developed a rotaxane-based computer chip with a 20 kB memory. The transistors on today’s computer chips are tiny, but gigantic when compared to molecule-based transistors. Researchers believe that molecular computer chips may revolutionise computer technology in the same way that silicon-based transistors once did.
Jean-Pierre Sauvage has also investigated rotaxanes’ potential. In 2000, his research group succeeded in threading two looped molecules together, forming an elastic structure that is reminiscent of the filaments in a human muscle (figure 5). They’ve also built something that can be likened to a motor, where the rotaxane’s ring spins alternately in different directions.
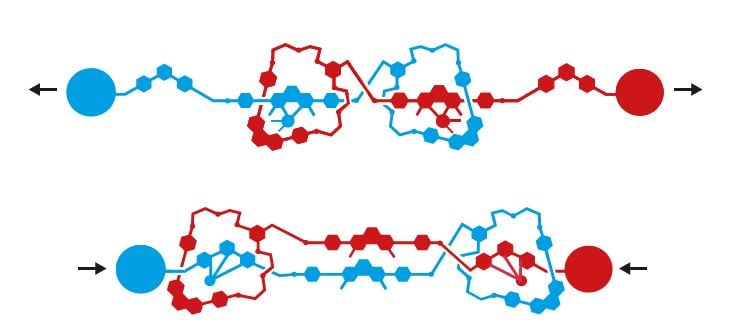
Producing motors that continually spin in the same direction has been an important goal for the art of molecular engineering. Many different attempts were made in the 1990s, but first across the line was Dutchman Bernard (Ben) L. Feringa.
Ben Feringa builds the first molecular motors
Just like Fraser Stoddart, Ben Feringa was raised on a farm and was attracted to chemistry by its endless opportunities for creativity. As he expressed it in one interview: “Perhaps the power of chemistry is not only understanding, but also creating, making molecules and materials that never existed before …”
In 1999, when Ben Feringa produced the first molecular motor, he used a number of clever tricks to get it to spin in one and the same direction. Normally, molecules’ movements are governed by chance; on average, a spinning molecule moves as many times to the right as to the left. But Ben Feringa designed a molecule that was mechanically constructed to spin in a particular direction (figure 6).
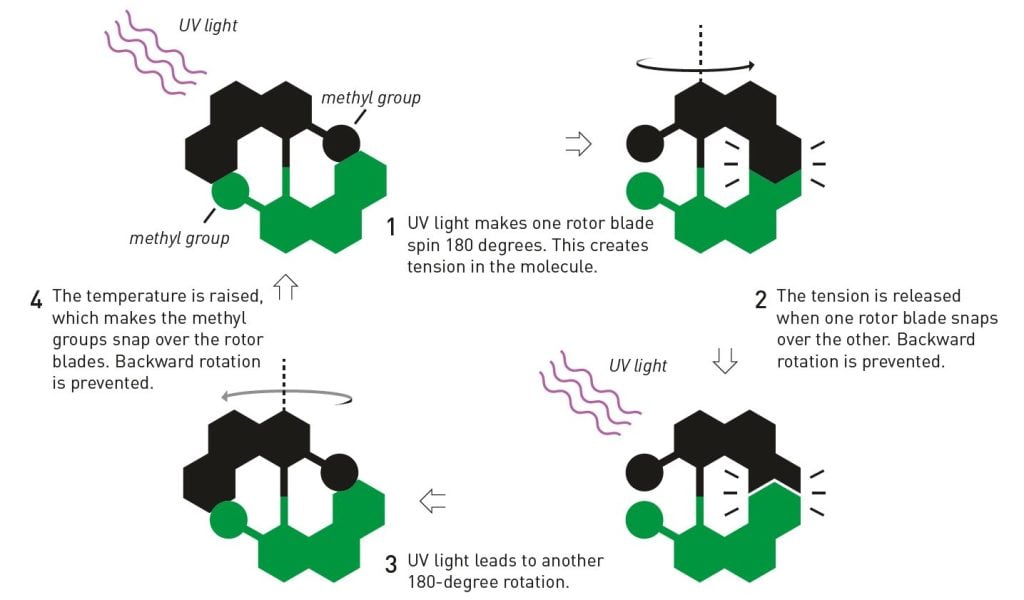
The molecule was composed of something that can be likened to two small rotor blades, two flat chemical structures that were joined with a double bond between two carbon atoms. A methyl group was attached to each rotor blade; these, and parts of the rotor blade, worked like ratchets that forced the molecule to keep rotating in the same direction. When the molecule was exposed to a pulse of ultraviolet light, one rotor blade jumped 180 degrees around the central double bond. Then the ratchet moved into position. With the next light pulse, the rotor blade jumped another 180 degrees. And so it continued, round and round in the same direction.
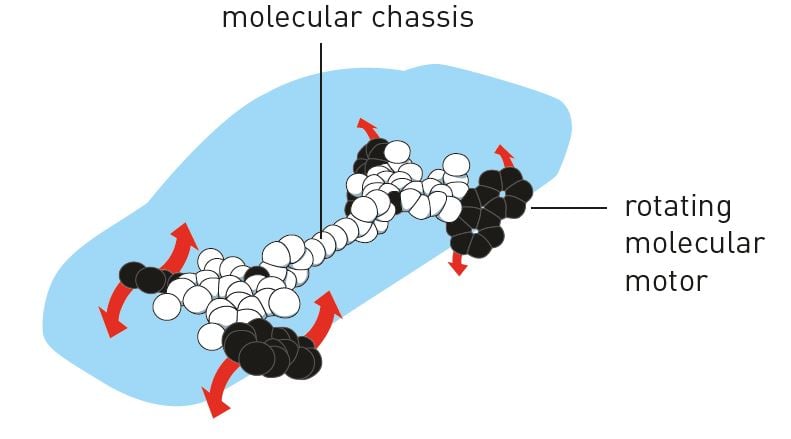
The first motor wasn’t exactly fast, but Feringa’s research group has optimised it. In 2014 the motor rotated at a speed of 12 million revs per second. In 2011, the research group also built a four-wheel drive nanocar; a molecular chassis held together four motors that functioned as wheels. When the wheels span, the car moved forward over a surface (figure 7).
A molecular motor spins a small glass cylinder
In another striking experiment, Ben Feringa’s research group has used molecular motors to spin a 28 micrometre long glass cylinder (10,000 times bigger than the molecular motors). In the experiment they incorporated the motors into a liquid crystal (a fluid with a crystalline structure). Only one per cent of the liquid crystal consisted of molecular motors but, when the researchers started them spinning, the motors changed the structure of the liquid crystal as they span. When the researchers placed the glass cylinder on top of the liquid crystal, it rotated due to the movement provided by the motors (a film of this process can be downloaded via: www.nature.com/nature/journal/v440/n7081/suppinfo/440163a.html).
A molecular toolbox to build upon
The groundbreaking steps taken by Jean-Pierre Sauvage, Fraser Stoddart and Ben Feringa in developing molecular machinery have resulted in a toolbox of chemical structures that are used by researchers around the world to build increasingly advanced creations. One of the most striking examples is a molecular robot that can grasp and connect amino acids. This was built in 2013 with a rotaxane as its foundation.
Other researchers have connected molecular motors to long polymers, so they form an intricate web. When the molecular motors are exposed to light, they wind the polymers up into a messy bundle. In this way, light energy is stored in the molecules and, if researchers find a technique for retrieving this energy, a new kind of battery could be developed. The material also shrinks when the motors tangle the polymers, which could be used to develop sensors that react to light.
Away from equilibrium – towards a new and vibrant chemistry
An important part of the development that has resulted in the Nobel Prize in Chemistry 2016 is that researchers have driven molecular systems away from what is called equilibrium. All chemical systems strive for equilibrium – a lower energy state – but this is somewhat of a stalemate. We can take life as an example. When we eat, the body’s molecules extract the energy from the food and push our molecular systems away from equilibrium, to higher energy levels. The biomolecules then use the energy to drive the chemical reactions necessary for the body to work. If the body was in chemical equilibrium, we’d be dead.
Just like the molecules of life, Sauvage’s, Stoddart’s and Feringa’s artificial molecular sytems perform a controlled task. Chemistry has thus taken the first steps into a new world. Time has clearly shown the revolutionary effect of miniaturising computer technology, whereas we have only seen the initial stages of what could result from the miniaturisation of machines. In terms of development, the molecular motor is at about the same stage as the electric motor was in the 1830s, when researchers proudly displayed various spinning cranks and wheels in their laboratories without having any idea that they would lead to washing machines, fans and food processors.
So, 32 years after Feynman’s visionary lecture, we can still only guess at the thrilling developments ahead of us. However, we do have a definite answer to his initial question – how small can you make machinery? At least 1,000 times thinner than a strand of hair.
Links and further reading
Book chapter
Sauvage, J.-P., Duplan, V. and Niess, F. (2016) Contractile and Extensile Molecular Systems: Towards Molecular Muscles. In R. M. Izatt (Ed.) Macrocyclic and Supramolecular Chemistry: How Izatt-Christensen Award Winners Shaped the Field. (s. 444-464). John Wiley & Sons, Inc.
Articles
Capecelatro, A.N. (2007) From Auld Reekie to the City of Angels, and all the Meccano in between: A Glimpse into the Life and Mind of Sir Fraser Stoddart. The UCLA USJ., 20,1-7.
Stoddart, J.F. (2009) The Master of Chemical Topology. Chem. Soc. Rev., 38,1521-1529.
Weber, L. and Feringa, B.L. (2009) “We Must be Able to Show How Science is Benefcial to Society.” Chimia, 63 (6),352-356.
Feringa, B.L. (2011) Ben L. Feringa. Angew. Chem. Int. Ed., 50, 1470-1472.
Peplow, M. (2015) The Tiniest Lego: a tale of nanoscale motors, rotors, switches and pumps. Nature., 525, 18-21.
Videos
NorthwesternU (2008, May 28). Nanotechnology Town Hall Meeting – Sir J. Fraser Stoddart.
https://www.youtube.com/watch?v=oOVXeRHnTAg
Francis Villatoro (2011, Nov. 9). A four-wheeled molecule moving on a metal surface.
https://www.youtube.com/watch?v=I5JgJsjq3Q4
Elsevier Journals (2016, Sept. 7). Tetrahedon Prize 2016.
https://www.youtube.com/watch?v=F-HNDwZrISA
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2016 to
JEAN-PIERRE SAUVAGE
Born 1944 in Paris, France. Ph.D. 1971 from the University of Strasbourg, France. Professor Emeritus at the University of Strasbourg and Director of Research Emeritus at the National Center for Scientifc Research (CNRS), France.
https://isis.unistra.fr/laboratory-of-inorganic-chemistry-jean-pierre–sauvage
SIR J. FRASER STODDART
Born 1942 in Edinburgh, UK. Ph.D. 1966 from Edinburgh University, UK. Board of Trustees Professor of Chemistry at Northwestern University, Evanston, IL, USA.
http://stoddart.northwestern.edu
BERNARD L. FERINGA
Born 1951 in Barger-Compascuum, the Netherlands. Ph.D.1978 from the University of Groningen, the Netherlands. Professor in Organic Chemistry at the University of Groningen, the Netherlands.
www.benferinga.com
”for the design and synthesis of molecular machines”
Science Editors: Olof Ramström, Gunnar von Heijne and Sara Snogerup Linse, the Nobel Committee for Chemistry
Text: Ann Fernholm
Illustrations: © Johan Jarnestad/The Royal Swedish Academy of Sciences
Editor: Ann Fernholm
Translation: Clare Barnes
© The Royal Swedish Academy of Sciences
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
