Award ceremony speech
Presentation Speech by Professor Ingvar Lindqvist of the Royal Academy of Sciences
Translation from the Swedish text
You Majesties, Your Royal Highnesses, Ladies and Gentlemen,
Henry Taube has been awarded the 1983 Nobel prize in chemistry for his studies of the mechanisms of electron transfer-reactions, particularly of metal complexes. I will not, during these few minutes, try to give a survey of the rich scientific production of Taube. Instead, I will choose one chemical reaction and attempt to show how our way of looking at this and similar reactions has changed drastically thanks to Taube.
Chemistry is a science which is rapidly aging. Most of the chemical investigations, basic or applied, which were published fifty years ago are to-day forgotten, not because they were wrong, but because our outlook of the science chemistry has changed so much during this time. Some few contributions are, however, still alive because they have determined in a decisive way our ideas about the fundamental relations in chemistry.
It must be in the spirit of Alfred Nobel that works of this type should be awarded the prize, each in its time. It can then be remembered that these new ways of thinking are in the long run also of utmost importance for applied chemistry, although it might be difficult to point directly to their usefulness at the occasion when the prize is awarded. Starting with Svante Arrhenius, who got the Nobel prize 1903, I will show how Taube fits in with this line of “new thinkers”. Arrhenius got the prize because he convinced his contemporary chemists that salts in aqueous solutions exist as positive and negative ions and not as neutral molecules. The reaction which I will discuss could, according to Arrhenius, be described as follows: Trivalent cobalt ions oxidize bivalent chromium ions and thereby form bivalent cobolt ions and trivalent chromium ions. The net result is an electron transfer between two positively charged ions. Arrhenius did not speculate further in molecular terms about the nature of the ions in the solution.
It was another Nobel prize winner (1913), Alfred Werner, who carried the development further in a decisive way when he proved that metal ions in solution are in many cases surrounded by a fixed number of neighbouring negative ions or neutral molecules. He also suggested that these neighbours are arranged in a certain way, e.g., in the corners of an octahedron if they are six in number. If Werner had known what we know to-day thanks to Taube, about the reaction I am discussing, he would have expressed the conditions in the following way: A trivalent cobolt ion surrounded by live ammonia molecules and one chloride ion is reacting with a bivalent chromium ion which probably is surrounded by six water molecules. During the reaction there are formed a bivalent cobalt ion surrounded by five ammonia molecules and one water molecule and a trivalent chromium ion surrounded by live water molecules and one chloride ion. The electron transfer is thus connected with a chloride ion transfer from cobolt to chromium. This description is certainly much more complete and gives a clear picture of the conditions before and after the reaction. It does not tell anything, however, about how the reaction has taken place.
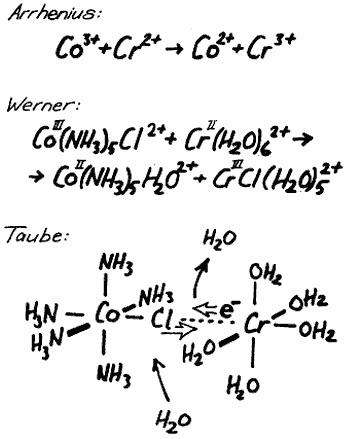
Taube has now proceeded one step further and has shown us exactly how the reaction occurs. The first step is the formation of a larger complex where the chromium ion is attached to the cobolt ion via the chloride ion which thus functions as a bridge between the two metal ions. This requires that a water molecule leaves the chromium ion to give place for the bridging chloride ion. The chromium ion will thus be surrounded by live water molecules and the bridging chloride ion. It is only when this bridge has formed that the electron transfer can take place making the cobalt ion bivalent and the chromium ion trivalent. Finally the bridge is opened and the chloride ion follows the trivalent chromium ion while the cobolt ion must take up a molecule of water to replace the chloride ion. In an impressive series of investigations Taube has developed and refined this idea and it will in the future be a natural part of the set of paradigms of chemistry, which thus in a decisive way has been enriched by the contributions of Henry Taube.
Professor Henry Taube,
I have not tried, during these few minutes, to give a comprehensive presentation of all your outstanding contributions to inorganic chemistry, but have rather preferred to try to show the conceptual importance of one of your achievements, which is an essential part of that work for which you have been awarded the Nobel prize. It is an honour and a pleasure for me to extend to you the congratulations of the Royal Academy of Sciences and to ask you to receive your prize from the hands of His Majesty the King.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
