Press release

17 October 1984
The Royal Swedish Academy of Sciences has decided to award the 1984 Nobel Prize in chemistry to
Professor R. Bruce Merrifield, Rockefeller University, New York, USA,
for his development of methodology for chemical synthesis on a solid matrix.
Summary
R. Bruce Merrifield, Professor at Rockefeller University, has been awarded the Nobel Prize in chemistry for 1984 for his development of a simple and ingenious method for obtaining peptides and proteins. This method has created completely new possibilities in the field of peptide and protein chemistry, which is Merrifield’s own area of research, as well as in the field of nucleic acid chemistry, where other researchers have applied Merrifield’s ideas. Merrifield’s method has greatly stimulated progress in biochemistry, molecular biology, pharmacology and medicine. It is also of practical importance, both for the development of new drugs and for gene technology. Merrifield’s method involves binding the first of the many amino acid residues of which a protein is composed to a special substance called a polymer. Such a synthesis is more rapid than those achieved with earlier methods and the quantity of final product obtained is considerably greater.
Background information
The chemical reactions which take place in living organisms are not spontaneous, but require the involvement of catalysts. These catalysts are called proteins. The first step in the formation (biosynthesis) of a protein in a living organism is the construction of a linear molecule called a peptide and composed of a large number of amino acid residues. In general these peptides can be subsequently modified in a number of ways. There are about 20 amino acids which are commonly used in the biosynthesis of proteins and the number of possible variations is virtually unlimited. A number of hormones and other signal substances which regulate different life processes are also peptides, but these compounds contain considerably fewer amino acid residues than do proteins.
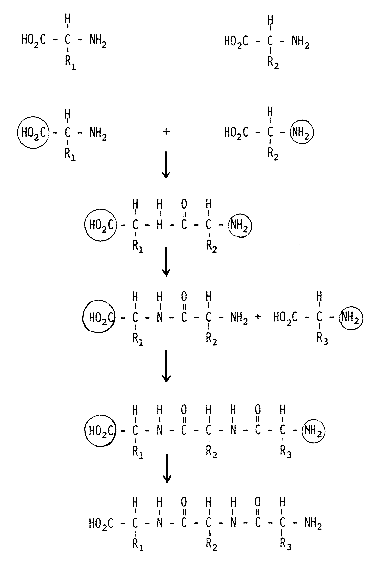
We know the detailed structure of a large number of proteins and peptides, thanks to the efforts of Frederick Sanger (Nobel Prizewinner in 1958) and Stanford Moore and William H. Stein (Nobel Prizewinners in 1972), among others. A very important contribution was also made by the Swedish researcher Pehr Edman, who unfortunately died relatively young but who left behind an automated method for determining peptide structure which is now used routinely throughout the scientific world. The chemical synthesis of peptides is an important task for organic chemists and the principle generally used today for such synthesis was developed a relatively long time ago by the Nobel Prizewinner in chemistry for 1902, Emil Fischer (Fig.1). All amino acids contain two typical functional groups, an acid (carboxylic) group and a basic (amino) group. When the amino group in one amino acid reacts with the carboxylic group in another, the resulting chemical bond gives rise to a dipeptide. In order for this reaction to take place in a controlled manner, the carboxylic group on amino acid 1 and the amino group in amino acid 2, groups which are not to be involved in the reaction, must be protected. If one of these protecting groups in the dipeptide is later selectively removed and reaction with a third amino acid containing one protected group carried out, a tripeptide is formed. And so forth. This approach is simple in theory, but difficult in practice. After each step the product must be separated from biproducts and unreacted starting material and loss of product is inevitable during such separation. When the Nobel Prizewinner in chemistry for 1955, Vincent du Vigneaud, synthesized the peptide hormone oxytocin, which is a nonpeptide, this was a very great step forward. To use this method for synthesizing peptides containing some 100 amino acid residues is truly an heroic task. If the yield in each synthetic step is 90%, which is a very good yield, then the overall yield after 100 steps would be 0.003%. In order to obtain measurable amounts of the final product the first steps of the synthesis would thus have to be conducted on a very large scale and the synthesis becomes very tedious.
Merrifield solved this problem in a manner which is both simple and ingenious (Fig. 2). He attaches the first amino acid through its carboxylic group to a solid polymer. After each synthetic step, byproducts and remaining starting materials can thus be removed by filtration and washing the polymer. Only when the entire synthesis has been completed is the peptide removed from the polymer. The advantages of this method are very considerable. Through the replacement of a complicated isolation procedure for each intermediate product with a simple washing procedure much time is saved. In addition, it has proven possible to increase the yield in each individual step to 99.5% or better, a result which cannot be attained using conventional synthetic approaches. In the example given above the final overall yield would thus be increased from 0.003% to 61%. Finally, this method is also suitable for automation and automatic peptide synthesizers are now commercially available.
Merrifield’s methodology has brought about a revolution in peptide and protein chemistry and thousands of different peptides have now been synthesized using this approach. One milestone in this respect was the synthesis of an active enzyme, ribonuclease A, by Merrifield himself and his coworkers.
Merrifield’s idea of performing a multistep synthesis with a compound attached to a solid matrix as the starting material has also been used in other areas. The most important of these is undoubtedly the synthesis of oligonucleotides, which are needed in hybrid DNA research. In this case as well an automated apparatus which can be programmed to synthesize desired products has been constructed. Although Merrifield has not worked in this area himself, it is clearly his ideas which have found a new application here.
Merrifield’s methodology is a completely new approach to organic synthesis. It has created new possibilities in the research fields of peptide-protein chemistry and nucleic acid chemistry It has greatly stimulated progress in biochemistry, molecular biology, pharmacology and medicine. It is also of great practical importance, both for the development of new drugs and for gene technology.
Figure 1. Synthesis of a tripeptide using conventional methodology. HO2C- is a carboxylic group, NH2- an amino group. A circle around a group indicates that it is protected and, thus, cannot react.
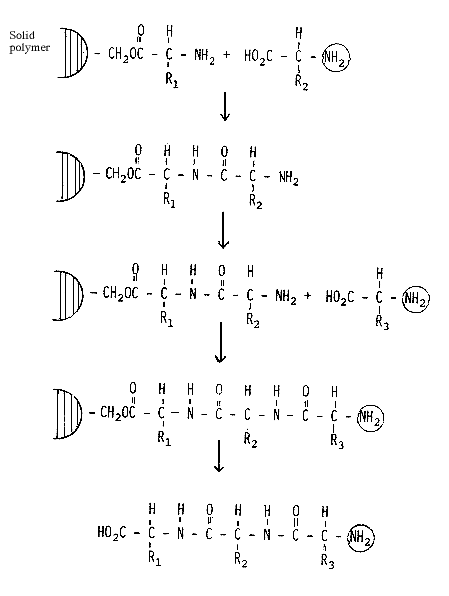 |
Figure 2. Synthesis of a tripeptide using Merrifield´s methology.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.