George A. Olah
Biographical
 I was born in Budapest, Hungary, on May 22, 1927 the son of Julius Olah and Magda Krasznai. My father was a lawyer and to my best knowledge nobody in my family before had interest in science. I grew up between the two world wars and received a rather solid general education, the kind middle class children enjoyed in a country whose educational system had its roots dating back to the Austro-Hungarian Monarchy. I attended a Gymnasium (a combination of junior and senior high school) at one of the best schools in Budapest run by the Piarist Fathers, a Roman Catholic order. A strict and demanding curriculum heavily emphasizing the humanities included 8 years of Latin, with German and French as other obligatory languages. Although we had an outstanding science teacher who later became a professor of physics in the University of Budapest I can not recollect any particular interest in chemistry during my school years. My main interest was in the humanities, particularly history, literature, etc. I was (and still am) and avid reader and believe that getting attached too early to a specific field frequently shortchanges a balanced broad education. Although reading the classics in Latin in school may be not as fulfilling as it would be at a more mature age, few scientists can afford the time for such diversion later in life.
I was born in Budapest, Hungary, on May 22, 1927 the son of Julius Olah and Magda Krasznai. My father was a lawyer and to my best knowledge nobody in my family before had interest in science. I grew up between the two world wars and received a rather solid general education, the kind middle class children enjoyed in a country whose educational system had its roots dating back to the Austro-Hungarian Monarchy. I attended a Gymnasium (a combination of junior and senior high school) at one of the best schools in Budapest run by the Piarist Fathers, a Roman Catholic order. A strict and demanding curriculum heavily emphasizing the humanities included 8 years of Latin, with German and French as other obligatory languages. Although we had an outstanding science teacher who later became a professor of physics in the University of Budapest I can not recollect any particular interest in chemistry during my school years. My main interest was in the humanities, particularly history, literature, etc. I was (and still am) and avid reader and believe that getting attached too early to a specific field frequently shortchanges a balanced broad education. Although reading the classics in Latin in school may be not as fulfilling as it would be at a more mature age, few scientists can afford the time for such diversion later in life.
After graduating from high school and having survived the ravages of war in Budapest and realizing the difficulties facing life in a small and war torn country, I started to study chemistry upon entering university, being attracted by the wide diversity it offered.
Classes at the Technical University of Budapest were relatively small. We probably started with a class of 70 or 80, whose numbers were rapidly pared down during the first year to maybe half by rather demanding “do or die” oral examinations, where the ones who failed could not continue. This was a rather cruel process, because laboratory facilities were so limited that only few could be accommodated. At the same time the laboratory training was thorough. For example, in the organic laboratory we did some 40 Gatterman preparations. It certainly gave a solid foundation.
Organic chemistry particularly intrigued me and I was fortunate later to become a research assistant to Professor Geza Zemplen, the senior professor of organic chemistry in Hungary, who himself was a student of Emil Fischer in Berlin. He established in Hungary a reputable school in organic chemistry. As Fischer, he too expected his students to pay their own way and even paying for the privilege to work in his laboratory. Becoming an assistant to him although meant no remuneration but also no fee. Zemplen had a formidable reputation, and working for him was quite an experience. He also liked partying and these remarkable events in neighboring pubs lasted frequently for days. Certainly one’s stamina developed through these experiences.
Zemplen was a carbohydrate chemist, much interested in glycosides. Early in our association it became clear that my ideas and interest were not always closely matching his. When I suggested that fluorine containing carbohydrates may be of interest in coupling reactions, his reaction was not unexpectedly very negative. To try to pursue fluorine chemistry in post-war Hungary was indeed far fetched. Eventually, however, he gave in. Even basic chemicals needed for the work, such as HF, FSO3H or BF3 were non-existent and I made them myself, with enthusiastic help by some of my early associates (A. Pavlath, S. Kuhn). Laboratory space, particularly hoods (the kind exhausted only by draft caused by a gas burner causing warm air to raise and take some of the obnoxious fumes through a chimney) was very scarce and even by the time I became an assistant professor it was not welcome to “pollute” more important conventional work. However, the Institute which was on the second floor of the chemistry building, had in the back an open balcony, used to store chemicals. In one of his unexpected gestures Zemplen agreed that I can have the use of this balcony. With some effort we enclosed it, installed two old hoods and were soon in business in what was referred to as the “balcony laboratory”. I am not sure that Zemplen even set foot in it. We enjoyed, however, our new quaters and the implicit understanding that our fluorine chemistry and related study of Friedel-Crafts reactions and their intermediates was now officially tolerated.
Some of my publications in the early 50s from Hungary caught the eye of Hans Meerwein. It is still a mystery to me how he came to read them in a Hungrian journal, although there also was a foreign language edition of the Hungarian Chimica Acta. Anyhow, I received an encouraging letter from him and we followed up correspondence (not easy at a time in completely isolated Hungary). He must have sympathized with my difficulties because one day through his efforts I received a cylinder of boron trifluoride. What a precious gift it was!
The Hungarian educational system after the Communist takeover was realigned according to the Soviet example. University research was deemphasized and research institutes were established under the auspices of the Academy of Sciences. I was invited to join the newly established Central Chemical Research Institute of the Hungarian Academy of Sciences in 1954 and was able to establish a small research group in organic chemistry, housed in temporary laboratories of an industrial research institute. With my group, which by now also included my wife, we were able to expand our work and made the best of our possibilities. In October 1956 Hungary revolted against the Soviet rule, but the uprising was soon put down by drastic measures and much loss of life. Budapest was again devastated and the future looked rather dim. In November-December 1956 some 200,000 Hungarians, mostly of the younger generation fled their country. With my family and much of my research group we also decided to follow this path and look for a new life in the West.
I married in 1949 Judith Lengyel, the best thing ever to happen to me in my life. We knew each other from our early youth and are happily married now for more than 45 years. Judy worked initially as a technical secretary at the Technical University. After we were married she enrolled to study chemistry. She probably rightly recalls that I was entirely responsible for this step and she only agreed to get along with her single minded husband who seemed to believe that there is little in life outside chemistry. From my point of view for husband and wife to closely understand each other’s work and may even work together was most desirable. Our older son George John was born in Budapest in 1954. After we fled Hungary in early December of 1956, we reached late in December London where my wife had relatives. We subsequently moved on in the spring of 1957 to Canada, where my mother-in-law lived in Montreal after the war. During our stay in London for the first time I was able to establish personal contact with some of the organic chemists, whose work I knew and admired from the literature. I found them most gracious and helpful. In particular Christopher Ingold and Alexander Todd extended efforts on behalf of a young, practically unknown Hungarian refugee chemist in a way which I never forget and for which I am always grateful.
Dow Chemical, with its home base at Midland, Michigan was establishing at the time a small exploratory research laboratory 100 miles across the border in Sarnia, Ontario where its Canadian Subsidaries major operations were located. I was offered a position to join this new laboratory and they also hired two of my original Hungarian Collaborators, including Steven Kuhn. We moved to Sarnia in late May of 1957. As our moving expenses where paid we checked in two cardboard boxes containing all of our worldly possessions unto the train from Montreal and started our new life. Our younger son Ronald Peter was born in Sarnia in 1959. There was no possibility for Judy to continue her career at the time. Sacrificing her own career she devoted herself to bring up our children. She rejoined in our research only a decade later in Cleveland after I returned to academic life.
The Sarnia years at Dow were productive. It was during this period in the late 50’s that my initial work on stable carbocations was started. Dow was and is a major user of carbocationic chemistry, such as the Friedel-Crafts type manufacture of etylbenzene for styrene production. My work thus also had practical significance and helped to improve some industrial processes. In return I was treated well and given substantial freedom to pursue my own ideas. Eventually I was promoted to company Scientist, the highest research position without administrative responsibility.
In the spring of ’64 I transferred to Dow’s Eastern Research Laboratories in Framingham, Massachusetts established under Fred McLarrerty’s directorship. The laboratory was subsequently moved to Wayland, just outside Boston. In the summer of 1965 I was invited to join Western Reserve University in Cleveland, Ohio and returned to academic life as professor with the added responsibility of becoming also Department Chairman.
My Cleveland years were both scientifically and personally most rewarding. My wife Judy was able to rejoin me in our research and my research group grew rapidly. The chemistry departments of Western Reserve University and neighboring Case Institute of Technology were practically adjacent, separated only by a parking lot. It became obvious that it would make sense to join the two into a single, stronger department. We achieved this by 1967 with surprisingly little friction and I was asked to serve as the Chair of the joint department till things settled down. It was in 1969 that I was able to give up my administrative responsibility. As I worked hard my research never suffered during this period and as a matter of fact these were probably some of my most productive years.
After 12 years in Cleveland it was time again to move on. Our older son George was approaching the end of his college years and our younger son Ron who was finishing high school set his mind to go to Stanford. He convinced us that it should be nice for the whole family to resettle in California. Coincidentally, in the fall of 1976 Sid Benson, an old friend called me to find out whether I would be interested to join him at the University of Southern California in Los Angeles. After some visits to LA the challenge of trying to build up chemistry in a dynamic university and the attractiveness of life in Southern California convinced us to move. We fell in love with California and we still are. As USC had limited chemistry facilities, it was offered to establish a research institute in the broad area of hydrocarbon research and provide it with its own building and facilities. We moved in May of 1977. Some 15 members of my research group joined the move West. By arrangements worked out we were able to take with us most of the laboratory equipment, chemicals, etc. Two weeks after our arrival with some large moving vans we were back doing chemistry in temporary quarters, while our research institute was constructed. The Institute was established at USC with generous support by Mr. & Mrs. D.P. Loker, friends and great supporters of the University. The Institute was subsequently named after them. Don Loker passed away some years ago, but Katherine still chairs the Institute’s board. Through her and other friends’ generosity a wonderful new addition to our Institute is just completed doubling our space.
As rewarding as the Nobel Prize is personally to any scientist, I feel it is also recognition of all my past and present students and associates (by now numbering close to 200), who contributed over the years so much through their dedicated hard work to our joint effort. It also recognizes fundamental contributions by many colleagues and friends from around the world to a field of chemistry, which is not frequently highlighted or recognized.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Addendum, May 2005
It is frequently said that receiving the Nobel Prize put so many obligations and commitments on the winners that their scientific work inevitably suffers. I was much determined that this should not happen to me. Having received the prize at the age 67 also helped, as my lifelong habits were solidly developed. I was determined that the prize should not affect significantly my life and certainly not my research.
I feel that I mostly succeeded. The intervening years were very productive and in many ways most rewarding for my research. Helped by my dedicated younger colleagues and associates and by close collaboration with my colleague Professor Surya Prakash, I was able to not only to continue my research but to extend it into new challenging areas.
A significant part of my previous research was based on the study of positively charged carbon compounds (carbocations) using superacids and their chemistry. The extremely strong acids I used and explored turned out to be many billions or even trillions of times stronger than previously recognized “strong” acids such as concentrated sulfuric acid.
The vastly increased acidity of superacidic systems resulted in the significant new field of superacid chemistry. In the last decade I asked myself whether a similar but more general approach could be used to produce in general electrophiles (electro deficient reagents) of greatly enhanced electron reactivity.
This resulted in the development of the concept of superelectrophilic activation and the study of superelectrophiles, i.e. electrophiles of greatly enhanced reactivity compared with previously known related electrophilic reagents and systems.
The concept of superelectrophiles thus emerged from my previous studies on superacidic carbocation and onium ion systems. It is based on the realization that a variety of electrophiles capable of further interaction (coordination) with strong Bronsted or Lewis acids can be greatly activated by them. Examples include onium and carboxonium ions, acyl cations, halonium, azonium, carbozonium ions, even certain substituted carbocations and the like. This activation produces what is now known as superelectrophiles, that is, electrophiles of doubly electron-deficient (dipositive) nature whose reactivity significantly exceeds that of their parents. Superelectrophiles are the de facto reactive intermediates of many electrophilic reactions in superacidic systems (including those involving solid superacids) and even some enzymatic systems and should be differentiated from energetically lower-lying, thus much more stable intermediates, which frequently are observable and even isolable but are not necessarily reactive enough without further activation.
Examples of some superelectrophiles so far studied and their parents are
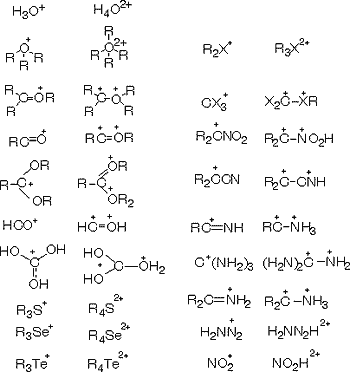
It should be recognized that superelectrophilic reactions frequently proceed with only “electrophilic assistance” (solvation, association) by the superacids without forming distinct dipositive intermediates. Protosolvolytic activation of electrophiles should always be considered in this context.
Another area of my post-Nobel research, that turned into a major continuing effort, evolved from the realization that our hydrocarbon resources, the marvelous gift of nature in the form of petroleum oil, natural gas and coal, are finite and not renewable.
The rapidly growing world population, which was 1.6 billion at the beginning of the twentieth century, has now well exceeded 6 billion. Even if mankind increasingly would exercise population control, by mid-century we will reach around 9.5-10 billion. This inevitably puts enormous pressure on our resources, not the least on our energy resources. For its survival, mankind needs not only food, clean water, shelter clothing, etc. but also energy. Since the cave man first managed to keep light and fire, our early ancestors burned wood and subsequently other natural sources. The industrial revolution was fueled by coal. The twentieth century added oil and natural gas and introduced atomic energy.
When fossil fuels such as coal, oil, or natural gas (i.e. hydrocarbons) are burned in power plants to generate electricity or to heat our homes and fuel our cars and airplanes, they form carbon dioxide and water. Thus, they are used up and are nonrenewable (at least on the human time scale). To find ways to replace our diminishing natural resources hydrocarbons will to be made by ourselves in a renewable, economical, and environmentally adaptable, clean way. This represents a major challenge for mankind in the twenty-first century.
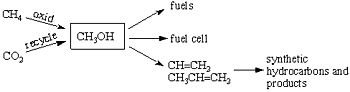
I have developed a promising new approach for solving not only our long range dependence on decreasing fossil fuels (oil, gas, and coal) but also at the same time to mitigate global climate change (warming) caused significantly by derived greenhouse gases such as carbon dioxide and methane. The approach is based on the use of methanol (CH3OH) as a way to store energy, as well as a convenient fuel and hydrocarbon source. Methanol as a fuel can also be directly used in the new fuel cell we developed jointly with the Jet Propulsion Laboratory of Caltech. It is also a raw material for synthetic (man made) hydrocarbons through its conversion to ethylene or propylene (by catalytic bimolecular dehydration i.e. 2CH3OH->CH2=CH2+2H2O). From these one can produce all the hydrocarbon fuels and products (from gasoline and diesel oil, to plastics, synthetic materials, pharmaceuticals, etc.), which are currently made from oil and natural gas. I call this new approach the “methanol economy”.
Currently methanol is still produced from fossil fuels, predominantly from natural gas through syn-gas (a mixture of CO and H2) by so-called Fisher-Tropsch chemistry, which is however a highly energy vasting process. We have developed new methods to convert still existing natural gas (methane) directly and efficiently to methanol. The true methanol economy, however, will do without natural gas, oil and coal as it is possible to produce methanol by the reaction of carbon dioxide with hydrogen. Exhaust gases from power plants and varied industrial emissions contain considerable amounts of carbon dioxide, which can easily be separated. Rather than just collecting carbon dioxide and storing it underground or at the bottom of the seas (as it is suggested) it can be used to produce methanol. Eventually, atmospheric carbon dioxide itself will be possible to be separated and converted to methanol. As atmospheric carbon dioxide is available to all people on the Earth this will enable mankind to liberate itself from dependence on fossil fuels. Substantial energy is of course necessary to generate the needed hydrogen for methanol production. This energy could come from safe nuclear power plants as well as all alternative energy sources such as sunlight, wind, geothermal, etc. At the same time, this approach will also diminish the danger of global warming by removing and recycling the rising carbon dioxide content of the atmosphere.
 |
I am fortunate to have retained my interest and drive to continue research with quite unabated energy. I published in the last decade years a number of books and monographs, notably: “Hydrocarbon Chemistry” (with Arpad Molnar,) 2nd revised ed., Wiley, 2003. “Onium Ions” (with Kenneth Laali, Qi Wang, and Surya Prakash,) Wiley, 1998. “Across Conventional Lines, selected papers of George Olah” (ed. with Surya Prakash), World Scientific Publishing, Singapore, 2003. “Carbocation Chemistry” (ed. with Surya Prakash), Wiley, 2004. “Beyond Oil and Gas: The Methanol Economy” (with Alain Goeppert and Surya Prakash), Wiley-VCH, 2005 (in preparation). I also published about 170 additional research papers and obtained some 15 patents.
For more biographical information, see:
Olah, George A., A Life Of Magic Chemistry: Autobiographical Reflections of a Nobel Prize Winner. Wiley-Interscience, New York, 2000.
George A. Olah died on 8 March 2017.
Copyright © The Nobel Foundation 2005Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
