Press release

12 October 1994
The Royal Swedish Academy of Sciences has decided to award the 1994 Nobel Prize in Chemistry to
Professor George A. Olah, University of Southern California, USA
for his contributions to carbocation chemistry.
Carbocations: from hypothetical intermediate products to well defined molecules
Most of us can recall from our high school chemistry courses that many so called inorganic compounds, like for example ordinary table salt, NaCl, could be regarded as built up from atoms or groups of atoms that are electrically charged. This table salt can be regarded as positively charged sodium ions (Na+) forming a bond with negatively charged chlorine ions (Cl–). To take another example; “glauber salt”, Na2SO4, can be thought of as two Na+ ions forming a bond with one SO42- ion.
While such electrically charged species – “ions” – are common in the world of inorganic compounds the opposite is true in the world of organic compounds, particularly in the case of the so called hydrocarbons. Hydrocarbons are compounds that are made up from only two types of elements – hydrogen (H) and carbon (C). Hydrocarbons constitute a very large and important group of organic compounds – for example most products from natural mineral oil are hydrocarbons. Although some hydrocarbons prepared by chemists around the turn of the century were thought to be ionic – e.g. a group of compounds formed from benzene and methane (“triarylmethane derivatives”) these were largely regarded as curiosities.
When some chemists in Britain (Ingold & Hughes) and Germany (Meerwein) in the 1920s and 1930s started detailed studies of how chemical reactions between organic molecules took place it, however, became apparent that positively charged hydrocarbons – what chemists call “carbocations” – actually could occur as very short lived (lifetimes of microseconds to nanoseconds) intermediates in the reactions.
Since these postulated “carbocation intermediates” were likely to be not only very short lived but also very reactive, it was generally assumed that one would never be able to prepare them in some quantities. Nor be able to study their properties with different physical techniques – e.g. NMR and infrared (IR) spectroscopy or X-ray diffraction – like one could do with normal uncharged hydrocarbons. But the direction of this field did change completely through the original and imaginative work by this years Nobel Prize laureate in Chemistry Professor George A. Olah.
In the early 1960s Olah and co-workers discovered that stable carbocations could be prepared through the use of a new type of extremely acid compounds – far stronger than “classical” acids like sulphuric acid, hydrochloric acid etc. These new acids – some of which were first described by the Canadian inorganic chemist, R. J. Gillespie – became generally known as “superacids”. A superacid can for example be prepared from hydrogen fluoride (HF) and antimony pentafluoride (SbF5).
Olah’s discovery completely transformed the scientific study of the elusive carbocations. Since the original discovery a large number of carbocations have been prepared and their properties studied in great detail. Olah has also shown how basic knowledge on superacids and carbocations can be applied to the facile synthesis of new and important organic compounds and that a number of small organic molecules, with widespread use as starting material in many large scale synthesis, can be produced in a simple and inexpensive way using superacids as catalysts. His work has resulted in new methods for the conversion of straight chain hydrocarbons (when used in combustion engines these have very low octane number and they are also difficult to degrade biologically) into branched hydrocarbons that have high octane numbers and are more easily biodegradable.
Olah’s scientific contributions have won widespread recognition among organic chemists and his work on carbocations now has a prominent position in all modern textbooks on organic chemistry.
Background
George A. Olah has through his research on the cations from carbon compounds (carbocations) in superacidic solvents and at low temperatures opened new avenues towards new and detailed knowledge of their structure and reactivity. His work has also led to the discovery of new reactions of considerable potential in the chemical industry and elsewhere.
In a chemical reaction, the molecules of the original material are converted into a final product. This most often occurs via very short-lived (10-10 – 10-6seconds) entities termed reactive intermediates. In many organic reactions these are carbocations. Since they are so short-lived they occur in such low concentrations that they cannot be directly observed with, for example, spectroscopy. Knowledge of their existence, structure, reactivity and so on has therefore been very incomplete.
It is important to understand how reactions proceed to be able to control them, intervening to obtain the products desired. This is especially important for the chemical industry. In his research, Olah endeavoured to give the short-lived carbocations a long life. It was necessary to get them to react more slowly with solvents and other nucleophilic molecules. (Nucleophiles are anions or solvents that have a free electron pair and that can attack a positive ion or a positively polarised atom in a molecule.) Olah found he could use solvents that were very little nucleophilic (e.g. S02, SO2ClF and SO2F2) and that therefore at low temperatures react slowly with carbocations. To produce carbocations he used what are termed superacids (acids that are stronger than 100% sulphuric acid).
Dissolving alkyl halides at low temperature in hydrogen fluoride-antimon pentafluoride (HF-SbF5), which is 1018 times stronger than 100% sulphuric acid, he managed for the first time to produce trivalent carbocations (carbenium ions) in such high concentrations and of such long life spans that, using nuclear magnetic resonance spectroscopy (NMR) and electron spectroscopy for chemical analysis (ESCA), he was able to study their structure, stability, properties and reactivity. Olah’s pioneering work has made it possible to observe carbocations directly with various spectroscopic methods and to gain detailed knowledge of their structure and reactivity.
Olah also found that superacids are so strong that they can even bind more hydrogen ions to simple hydrocarbons, forming pentacoordinated carbocations (carbonium ions). This has already had practical consequences in hydrocarbon chemistry, leading, for example, to new methods of isomerising hydrocarbons and synthesising higher hydrocarbons from methane.
Very many carbocations of great structural variation have now been studied. The results have brought many surprising and important contributions to our understanding of chemical bindings. Besides trivalent (tricoordinated) carbocations, carbocations with higher coordination – tetra, penta- and hexacoordination – have been generated and their structure determined. The old dogma of the tetravalency of carbon, a cornerstone of structural organic chemistry since the days of Kekulé in the 1860s, was thus destroyed.
History
During the 1920s, mainly through the research of C.-K Ingold (1893-1970, UK), the mechanisms of many organic reactions were elucidated. Two of the commonest and most widely used reactions in synthetic organic chemistry are nucleophilic substitution and elimination. In nucleophilic substitution the attacking reagent (the nucleophile) carries an electron pair to the substrate, using this pair to form the new bond while the leaving group departs with an electron pair. In elimination, two groups on adjacent carbon atoms are lost and an olefine (alkene) is formed. Depending upon the structure of the substrate, the solvent and a number of other factors, these reactions can occur in two stages. These are exemplified below with isopropyl chloride which, in the presence of the nucleophile Nu-, reacts to give a nucleophilic substitution product and/or an elimination product:
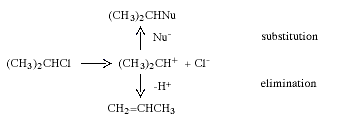
In the first stage the carbon-chlorine bond is split, and a short-lived carbenium ion is obtained. In the next step this rapidly reacts either with Nu- and gives the substitution product or transfers a proton to the solvent or Nu- to give propene.
The results of extensive kinetic and stereochemical investigations were consistent with mechanisms involving carbocations as intermediates. Carbenium ion structures and carbonium ion structures (non-classical ions) were suggested as hypothetical intermediates. To explain the results, it was also necessary to assume that the carbocations could often be associated with some negatively charged ion to give contact-ion pairs or solvent-separated ion pairs. A prominent figure in this later research was S. Winstein (1912-1969, USA).
The carbocations studied, however, were so short-lived (10-10– 10-6 seconds) that they could not be directly observed with spectroscopy. The picture of these important intermediates still remained incomplete.
Olah’s extremely important contribution lies in the methods he evolved for developing carbocations in high concentrations and under conditions which give them long life. To achieve this, he used solvents which were so extremely little nucleophilic that they did not attack carbocations. Such solvents are SO2, SO2ClF and SO2F2in which, at least at temperatures around -100°C, carbocations do have long life. To generate carbocations, Olah used various superacids including SbF5, which is a Lewis superacid, giving carbocations with e.g. alkyl halides. Others were Brønsted superacids such as HSO3F or the extremely strong superacids obtained by combining e.g. HSO3F or HF with SbF5. Magic Acid®, HSO3F:SbF5 and HF:SbF5 are 1018 times stronger than 100% sulphuric acid. Of these, Magic Acid® and H:SbF5 are so strong that they can completely protonate alcohols and olefines, thus giving carbocations in high concentrations. Temperatures between -78°C and -120°C are usually used. Especially 1H- and 13C- NMR-spectroscopic studies have given detailed knowledge of the structure, stability and reactivity of carbocations.
Investigations of the nucleophilic substitution reactions and rearrangements of 2-norbornyl derivatives led S.Winstein to suggest in the early 1950s that the intermediate carbocation was non-classical and contained a pentacoordinated carbon (C6 in Figure la coordinates to two hydrogen atoms besides C1, C2 and C5). This interpretation was questioned by H.C. Brown (1912, USA), who received the 1979 Nobel Prize in Chemistry for his development of boron compounds into important reagents in organic synthesis. Brown claimed that the 2-norbornyl cation did not have carbonium ion structure but was a carbenium ion that rapidly rearranged itself into itself, i.e. it was a rapidly equilibrating carbenium ion (Figure 1b).
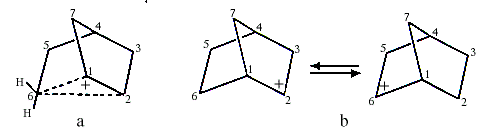 |
| Figure 1. a) Carbonium ion b) Equilibrating carbenium ions |
The ensuing scientific controversy lasted until about 1980. As the structures of carbonium ions were of great theoretical interest, the problem fascinated many leading physical organic chemists, yet despite great efforts and many ingenious experiments, no definitive solution was found until the 2-norbornyl cation could be directly studied with NMR-spectroscopy.
Olah and his co-workers finally observed the 2-norbornyl carbocation in a solution of SbF5-SO2ClF-SO2F2 at -158°C. Both 1,2-hydride shifts and more complicated rearrangements at this low temperature are slow enough not to disturb the interpretation of the NMR-spectra.
The spectra accorded completely with Winstein’s symmetrical bridged structure, with a pentacoordinated carbon (Figure la) and not with a rapidly equilibrating carbenium ion. Studies using electron-spectroscopy for chemical analysis (ESCA), developed by the Swedish Nobel laureate in physics for 1981, Kai Siegbahn, confirmed these conclusions.
Molecules containing pentacoordinated carbon atoms are no longer an exotic curiosity in organic chemistry. They have been found in inorganic compounds, organometallic compounds e.g. organolithium compounds, carboranes and other cluster compounds.
Superacids are so strong that they can protonate such extremely weak bases as the alkanes, as was shown by Olah and independently by H. Hogeveen. Thus, pentacoordinated carbonium ions have been obtained from methane higher alkanes and various cycloalkanes. Methane gives the methionium ion CH5+, which Olah has formulated as containing a three-centre, two-electron bond (Figure 2a, indicated with triangular dotted lines). Note that their junction does not represent an additional atom. Higher alkanes can be protonated both at C-H bonds (Figure 2b) and at C-C bonds (Figure 2c).
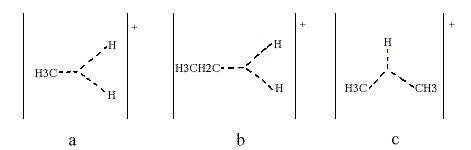
Olah found that for instance protonated isobutane decomposes to the t-butyl cation and molecular hydrogen. From the classical point of view, this is quite an unreasonable reaction; isobutane is oxidized by the proton to give the t-butyl cation and molecular hydrogen.
The protonation of saturated hydrocarbons in superacidic media has, through Olah’s work, already had practical consequences. It has led to a method for isomerising straight alkanes into branched alkanes of higher octane number. It has permitted the preparation of higher alkanes with methane as building block, illustrated below in the formation of ethane from methane. Superacid catalysis has also made it possible to crack heavy oils and to liquefy coal under surprisingly mild conditions.
![]()
Olah has recently shown that our most common electrophiles such as the acyl cation and the nitronium ion are protonated in superacidic media into doubly charged superelectrophiles. This leads to a dramatic increase in electrophilic reactivity. It is already obvious that Olah’s new chemistry has very broad and important applications.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
