The Nobel Prize in Chemistry 1995
Ozone in small amounts
If all atmospheric ozone were compressed to the pressure at the earth’s surface, the layer would be only 3 mm thick.
Even though ozone occurs in such small quantities it plays a fundamental role for life on earth.
Ozone for better and for worse
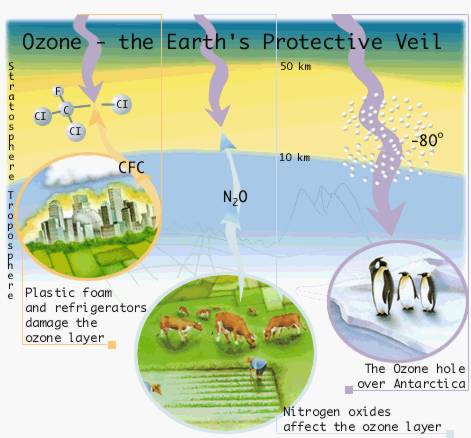
The atmosphere surrounding the earth contains small quantities of ozone, most of which is in the ozone layer in the stratosphere, 10-50 km above the earth’s surface.
Stratospheric ozone, together with molecular oxygen, absorbs most of the ultraviolet radiation from the sun. This prevents the radiation from reaching the earths surface where it can damage plants, animals and humans.
The ozone content in the lower layer of air (the troposphere, 0-10 km) has increased through the release of gaseous nitrogen oxides and hydrocarbons from vehicles, industrial plants and other sources. Ozone here can damage crops and people’s health and also contribute to raising the temperature at the surface – the “greenhouse effect”.
 |
|
|
Humans affect the earth’s environment by releasing substances that deplete the protective ozone layer. The Nobel laureates in Chemistry 1995, Mario Molina, Sherwood Rowland and Paul Crutzen, clarified the mechanisms for the chemical reactions involved. The results have led to extensive limitations on the release of ozone-damaging substances. Read more about the ozone layer and: |
Ozone (03) is formed in the stratosphere through the splitting of ordinary oxygen molecules (02) by ultraviolet radiation from the sun. The liberated oxygen atoms (O) react, through the mediation of some arbitrary molecule (M), with molecular oxygen as follows: |
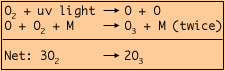 |
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
