Popular information
English
Swedish

The Nobel Prize in Chemistry 2002
The Nobel Prize in Chemistry for 2002 is being shared between scientists in two important fields: mass spectrometry (MS) and nuclear magnetic resonance (NMR). The Laureates, John B. Fenn and Koichi Tanaka (for MS) and Kurt Wüthrich (for NMR), have contributed in different ways to the further development of these methods to embrace biological macromolecules. This has meant a revolutionary breakthrough, making chemical biology into the “big science” of our time. Chemists can now rapidly and reliably identify what proteins a sample contains. They can also produce three-dimensional images of protein molecules in solution. Hence scientists can both “see” the proteins and understand how they function in the cells.
Revolutionary analytical methods for biomolecules
Why study biological macromolecules?
All living organisms – bacteria, plants and animals – contain the same types of large molecules, macromolecules, which are responsible for what we call life. Events in the cells are controlled by nucleic acids (such as DNA) that may be termed the cells’ “directors”, while the various proteins are the cells’ leading actors. Each protein has a biological function that may vary with its environment. The protein haemoglobin, for example, transports oxygen to all the cells in the body.
Protein research itself is not new, but proteomics, i.e. studies of how different proteins and other substances act together in the cell, is a relatively new field of research that has grown enormously in the past few years. As the gene sequences of more and more organisms have been mapped and the research frontier has advanced, new questions have cropped up: how can it be that man’s 30,000-or-so genes code for hundreds of thousands of different proteins? What happens if a gene is damaged or is missing? How do diseases such as Alzheimer’s or mad cow disease originate? Can the new chemistry be used to diagnose and treat more quickly the diseases that are threatening mankind?
To be able to tackle questions such as these chemists are in constant pursuit of more knowledge of proteins and how they function together with each other and with other molecules in the cells. This is because small variations in a protein’s structure determine its function. The next step is to study the dynamics: what do protein molecules look like at the very moment when they are interacting with one another? What happens at the decisive moments? To understand, we need to see.
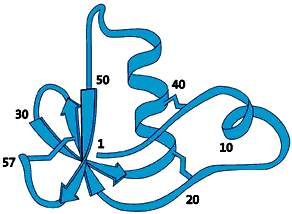
High resolution image (jpeg 223kb)
FIg 1. This protein consists of a long chain of amino acids that is pleated, folded and wound together like a ball of wool. It is this three-dimensional image of the protein one needs to achieve to be able to understand the function of that protein. This protein molecule, which was one of the first to have its structure determined with NMR, has a diameter of approximately one millionth of a centimetre
(10-8 m).
Mass spectrometry – a method of identifying molecules
Mass spectrometry now allows us to identify a substance in a sample, rapidly, on the basis of its mass. This technique has long been used by chemists on small and medium-sized molecules. The method is so sensitive that it is possible to trace very small quantities of each type of molecule. Doping and drug tests, foodstuff control and environmental analysis are examples of areas where mass spectrometry is now in routine use.
The foundations of mass spectrometry were already in place at the end of the nineteenth century. The first analyses of small molecules were reported in 1912 by Joseph J. Thomson. Several of the Nobel Prizes of the twentieth century depended directly on mass-spectrometric analysis. Examples are Harold Urey‘s discovery of deuterium (Nobel Prize in Chemistry 1934) and the discovery of the fullerenes, “carbon footballs” that gave Robert Curl, Sir Harold Kroto and Richard Smalley the Nobel Chemistry Prize in 1996.
The goal of using mass spectrometry for macromolecules as well long attracted the scientists. During the 1970s a number of successes were achieved in transferring macromolecules to ions in the gas phase, termed desorption technology. These have formed the basis for the revolution in this field during the past twenty years.
Macromolecules may be large in comparison with other molecules but we are nevertheless dealing here with incredibly small structures. Haemoglobin molecules, for example, have a mass of a tenth of a thousand-millionth of a thousand-millionth of a gram (10-19 g). How to weigh something that is so small? The trick is to cause the individual protein molecules to let go of each other and spread out as a cloud of freely hovering, electrically charged protein ions. A common method of subsequently measuring the mass of these ions – and hence identifying the proteins – is to accelerate them in a vacuum chamber where their time of flight (TOF) is measured. They “reach their targets” in an order determined partly by their charge and partly by their mass. The fastest ones are those that are lightest and have the highest charge.
Today there are two principles for causing proteins to transform into the gas phase without losing their structure and form, and it is the discoverers behind these methods that are being rewarded jointly with half the Nobel Prize in Chemistry. In one of these methods, of which John B. Fenn is the originator, the sample is sprayed using a strong electrical field to produce small, charged, freely hovering ions. The other method, instead, uses an intense laser pulse. If this is done under suitable conditions (as to the energy, structure and chemical environment of the sample) the test molecules take up some of the energy of the laser pulse and become released as free ions. The first person to show that this phenomenon, soft laser desorption, could be used for large molecules such as proteins was Koichi Tanaka.
Fenn’s contribution – hovering through spraying
During 1988 John B. Fenn published two articles that were to mean a breakthrough for mass spectrometry with “electrospray” for macromolecules. In the first, studies of polyethylene glycol molecules of unknown mass showed that the method could handle large molecule masses with high charges. The second publication reported the use of the method on medium-sized whole proteins as well. The release of ions is achieved by spraying the sample using an electrical field so that charged droplets are formed. As the water gradually evaporates from these droplets, freely hovering “stark naked” protein molecules remain. The method came to be called electrospray ionisation, ESI.
As the molecules take on strong positive charges, the mass/charge ratio becomes small enough to allow the substances to be analysed in ordinary mass spectrometers. Another advantage is that the same molecule causes a series of peaks, since each can take up a varying number of charges. While this complicates the pattern, at first confusing the researchers, it also gives information that makes identification easier.
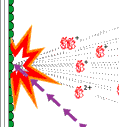 |
Fig 2. The principles for mass spectrometry of biomolecules. |
Tanaka’s contribution – hovering through blasting
At the same time exciting things were happening in another part of the world. At the Japanese Shimadzu instrument company in Kyoto, a young Japanese engineer, Koichi Tanaka, reported an entirely different technique for the first critical stage. At a symposium in 1987 and a year later in print, Tanaka showed that the protein molecules could be ionised using soft laser desorption (SLD). A laser pulse strikes the sample which, unlike in the spray method, is in a solid or viscous phase. When the sample takes up the energy from the laser pulse it is “blasted” into small bits. The molecules let go of one another, released as intact hovering molecule ions with low charge which are then accelerated by an electrical field and detected as described above by recording their time of flight. Tanaka was the first to demonstrate the applicability of laser technology to biological macromolecules. The principle is fundamental for many of today’s powerful laser desorption methods, particularly the one abbreviated MALDI (Matrix-Assisted Laser Desorption Ionisation) but also SELDI (Surface Enhanced Laser Desorption Ionisation) and DIOS (Direct Ionisation on Silicon).
Applications of mass spectrometry
Both electrospray ionisation (ESI) and soft laser desorption (SLD) have many areas of application. The sophisticated biochemical analyses now possible were but dreams a few years ago. Interactions between proteins are very important to study in order to understand the signal systems of life. Such non-covalent biomolecule complexes can be examined with ESI. The method is superior to other methods in the rapidity, sensitivity and identification of the actual interaction. Mass spectrometric analytical methods are relatively cheap, enabling them to spread quickly to laboratories all around the world. Today soft laser desorption (in the form of MALDI) and electrospray are standard methods for structure analyses of peptides, proteins and carbohydrates which make it possible to quickly analyse the protein content of intact cells and living tissue. The following examples of current fields of research gives a picture of the application versatility generated by this year’s Nobel Prize. Applications include:
Pharmaceuticals development
The early phase of pharmaceuticals development has undergone a paradigm shift. Combined with fluid separation, ESI-MS has made it possible to analyse several hundreds of compounds per day.
Malaria
Scientists have recently discovered new ways of studying the spreading of malaria. Early diagnosis is possible thanks to the soft laser desorption method. The oxygen-bearing part of human haemoglobin is used here to absorb the energy of the laser pulse.
Ovarian, breast and prostate cancer
New methods for early diagnosis of different forms of cancer have been reported at a rapid rate during the past year. By having a surface that cancer cells adhere to – and then analysing this with soft laser desorption – chemists can discover cancer faster than doctors can.
Foodstuff control
ESI technology has also made progress for small molecules. During the past few months we have learned that preparation of the food we eat can give rise to a number of substances hazardous to health, e.g. acrylamide which can cause cancer. With mass spectrometry, food is analysed rapidly at various stages of production. By modifying the temperature and the ingredients, the harmful substances can be avoided or minimised.
NMR for biological macromolecules
Where mass spectrometry gives answers to questions about e.g. a protein, such as “what?” and “how much?”. NMR in one sense answers the question “what does it look like?” Even the largest proteins are too small to be studied at sufficient resolution with any type of microscope. To be able to form a picture of what a protein really looks like, then, other methods must be used. NMR (Nuclear Magnetic Resonance) is one such method. By interpreting the peaks in an NMR spectrum one can draw a three-dimensional picture of the molecule being studied. One finesse is that the sample can be in a solution, in the case of proteins their natural environment in the cell.
Before the advent of NMR, X-ray crystallography was the only method available for determining the three-dimensional structure of the substance. In 1957 the first true three-dimensional structure of a protein, myoglobin, was presented. This was rewarded with a Nobel Prize in Chemistry to Max Perutz in 1962. X-ray crystallography is based on the diffraction of X rays in protein crystals, and has since contributed to a further series of Nobel Prizes. As a complement to X-ray crystallography, chemists long sought a method that would also function in a solution, i.e. an environment that better resembles the one the biomolecules surround themselves with naturally.
The physicists Felix Bloch and Edward Purcell discovered as early as in 1945 that some atom nuclei, through what is called their nuclear spin, absorb radio waves of a certain frequency when placed in a powerful magnetic field. This was rewarded with the Nobel Prize in Physics in 1952. A few years earlier it was discovered that the frequency for nuclear resonance depended not only on the strength of the magnetic field and the type of atom but also on the chemical environment of the atom. In addition, the nuclear spins of different nuclei could affect each other, generating fine structures, i.e. a further number of peaks in the NMR spectrum.
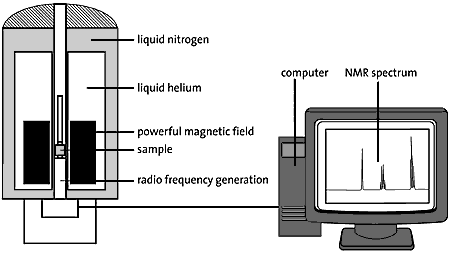
High resolution image (jpeg 120kb)
Fig 3. The sample to be examined is placed in a very strong magnetic field. The figure shows a super-conducting magnet cooled by liquid nitrogen and helium. Pulses of radio waves are sent into the sample which emits a radio wave “answer”. This response is analysed electronically and the result is an NMR spectrum.
The applicability of the NMR method was initially limited by its low sensitivity: it required incredibly concentrated solutions. But in 1966 the Swiss chemist Richard Ernst (Nobel Prize in Chemistry 1991) showed that this sensitivity could be increased dramatically if, instead of slowly varying the frequency, the sample was exposed to short and intense radio frequency pulses. He also contributed, during the 1970s, to the development of a way of determining what nuclei were adjacent to one another in a molecule, e.g. two atoms bound to each other. By interpreting the signals in an NMR spectrum it was thus possible to gain an idea of the appearance of the molecule, its structure. The method was successful for relatively small molecules but, for larger ones, it was hard to differentiate between the resonances of the different atom nuclei. An NMR spectrum of this kind could look like a grass lawn in section – thousands of peaks where it was impossible to decide which peak belonged to which atom. The scientist who finally solved this problem was the Swiss chemist Kurt Wüthrich.
Kurt Wüthrich – showed that NMR was possible for proteins
At the beginning of the 1980s, Kurt Wüthrich developed an idea about how NMR could be extended to cover biological molecules such as proteins. He invented a systematic method of pairing each NMR signal with the right hydrogen nucleus (proton) in the macromolecule (see fig. 4). The method is called sequential assignment and is today a cornerstone of all NMR structural investigations. He also showed how it was subsequently possible to determine pairwise distances between a large number of hydrogen nuclei and use this information with a mathematical method based on distance-geometry to calculate a three-dimensional structure for the molecule.
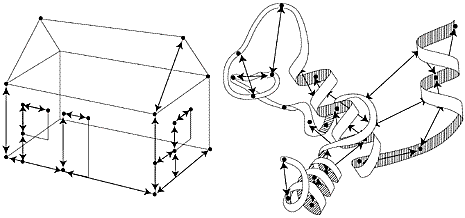 |
| High resolution image (jpeg 168kb) |
| Fig 4. If one knows all the measurements of a house one can draw a three-dimensional picture of the house. In the same way, by measuring a vast number of short distances in a protein it is possible to create a three-dimensional picture of its structure, as shown schematically in the figure. |
The first complete determination of a protein structure with Wüthrich’s method came in 1985. At present 15-20% of all the thousands of known protein structures have been determined with NMR. The structures of the others have been determined chiefly with X-ray crystallography; a few with other methods such as electron diffraction or neutron diffraction.
Areas of application for NMR with macromolecules
In many respects, the NMR method complements X-ray crystallography for structural determination. If the same protein is investigated with both methods, in the one case in solution and in the other crystallised, the same result is generally obtained, with the exception of certain superficial areas that are affected by the environment in both cases – in the crystals by the tightly packed protein molecules, in solution by the surrounding molecules of the solvent. While the strength of X-ray crystallography lies in being able to determine accurately really large three-dimensional structures, the NMR method has other unique advantages. The fact that the investigation takes place in a solution means that physiological conditions can be approximated. A particular strength of NMR is its ability to demonstrate unstructured and very mobile parts of a molecule. It is possible to elucidate the mobility, the dynamics, and how it varies along a protein chain. Isotope labelling can also be used to facilitate the identification of the atoms.
One example of NMR-determined protein structures comes from studies of the prion proteins involved in the development of a number of dangerous diseases such as mad cow disease (Nobel Prize in Medicine to Stanley Prusiner in 1997). Here Wüthrich and coworkers have shown with NMR methodology that the healthy form of prion proteins has two parts: approximately half of the protein chain assumes a well-ordered, fairly rigid three-dimensional structure in a water solution (121-231 in the picture below), while the other half is without structure and very mobile (23-120).
NMR can also be used in studies of structure and dynamics of other biological macromolecules such as DNA and RNA.
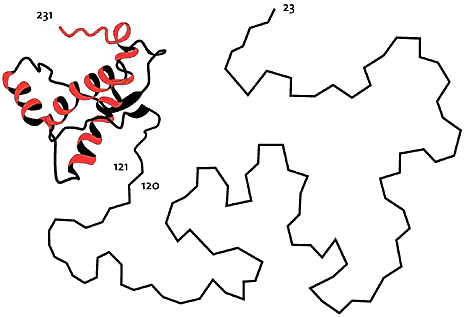
High resolution image (jpeg 171kb)
Fig 5. Structure of prion protein, determined with NMR. Half of the protein chain (23-120) is disordered and quite flexible in water solution.
NMR is also used in the pharmaceuticals industry to determine the structure, and hence the properties, of proteins and other macromolecules that can be interesting target molecules for new pharmaceuticals. Pharmaceutical molecules are designed to fit into the structure of the protein – like a key in a lock. The perhaps most important industrial use of NMR is in the search for small potential pharmaceutical molecules that can interact with a given biological macromolecule. If the small molecule binds to the large one, the NMR spectrum of the large molecule is normally changed. This may be used to “screen” a large number of pharmaceuticals candidates at an early stage of the development of a new drug.
| The Laureates | |
| John B. Fenn Virginia Commonwealth University Dept. of Chemistry 1001 W. Main St. P.O. Box 842006 Richmond, VA 23284-2006 USA www.has.vcu.edu/che/people/fenn.html |
US citizen. Born 1917 (85 years) in New York City, USA. PhD in chemistry 1940 and Professor at Yale University 1967–1987. Professor Emeritus 1987 at Yale University, Connecticut, USA. Since 1994 Professor at Virginia Commonwealth University, Richmond, Virginia, USA. |
| Koichi Tanaka Shimadzu Corp. 1. Nishinkokyo Kuwabaracho Nakagyou-ku Kyoto 604-8511 Japan www.shimadzu.com |
Japanese citizen. Born 1959 (43 years) in Toyama City, Japan. B. Eng 1983 at Tohoku University, Japan. R&D engineer at Life Science Business Unit, Analytical & Measuring Instruments Division, Shimadzu Corp., Kyoto, Japan. |
| Kurt Wüthrich Swiss Federal Institute of Technology Zürich ETH Hönggerberg, HPK CH-8093 Zürich Schweiz and The Scripps Research Institute 10550 North Torrey Pines Rd, La Jolla, CA 92037 USA www.mol.biol.ethz.ch/wuthrich www.scripps.edu/mb/wuthrich/people/kw/kw.html |
Swiss citizen. Born 1938 (64 years) in Aarberg, Switzerland. PhD in inorganic chemistry 1964 at The University of Basel. Since 1980 Professor of Molecular Biophysics at ETH, Zürich, Schweiz. Visiting Professor of Structural Biology at The Scripps Research Institute, La Jolla, California, USA. |
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
