Yves Chauvin
Biographical

I was born on 10 October 1930 in Menin (Menen in Flemish) in western Flanders, on the border between Belgium and France. My parents were French. To be more precise, they were not only both from the Tours region, but also descended from long-established families in the little village of Beaumont-la-Ronce. I used to spend my holidays there in my grandparents’ large family house, with my numerous cousins. When I die, I am going to be buried in the village cemetery. My grandmother was fond of painting and playing the piano. She had been given lessons by Emmanuel Chabrier, who used to spend the summer months in nearby Membrolle. He said in his correspondence that he did not much care for his pupils on the whole and my grandmother found him very strict. Most of my ancestors were small farmers. My father was an electrical engineer. After three years of military service and quite a difficult time in the First World War, he was sent by the company that had taken him on after his demobilisation to Ypres and then Menin, to work on rebuilding (building) the electricity network in this war-ravaged province. I remember him always being very motivated and working very long hours. He was sent to war again in 1939 and taken prisoner. There were 5 brothers and sisters in the family and we had quite a strict upbringing. From my bedroom, I looked out over our large garden (roses and vegetables) and the Lys river, which at that particular point separates France from Belgium and was where the flax was retted in tanks then dried in little bundles in a field (what a foul smell!). I watched the barges go past, towed by horses or even by men. It was a fascinating sight: the man pulling the rope remained bent over and unmoving for several minutes before the barge started to move. I also remember Vauban’s fortifications in this land of invasions, and the smell of roasted chicory … I still have many paintings of Flanders that my father bought from contemporary painters – somewhat classical, but not devoid of charm. I went to pre-school in Flanders and then the French primary school, which meant that I crossed the border every day. I then continued my secondary and higher education in various towns. During the war, I managed to come through the bombings unscathed, though sometimes only just. The war taught me to eat what there was; I am still not a fussy eater, although I do enjoy good food. To be perfectly truthful, I was not a very brilliant student, even at chemistry school. I chose chemistry rather by chance, because I firmly believed (and still do) that you can become passionately involved in your work whatever it is. Various circumstances, mainly to do with my military service, prevented me from doing a PhD and I have often regretted it, though you do need to choose the “right” supervisor in the “right” discipline – no easy task when you are totally inexperienced.
So I took a job in industry, but the fact that process development consisted primarily of copying what already existed, with no possibility of exploring other fields, prompted me to resign. Furthermore, I discovered that this was a very common attitude among managers. They are afraid of anything new: “Do what everyone else does and change as little as possible: at least we know it will work.” It is the opposite of my way of thinking, which, I must admit, is a bit of an obsession! I have often got into arguments about it. My motto is more, “If you want to find something new, look for something new!” There is a certain amount of risk in this attitude, as even the slightest failure tends to be resounding, but you are so happy when you succeed that it is worth taking the risk.
The whole contradiction of research (whether applied or fundamental) generally lies in the fact that we have to start out with the knowledge handed down by our predecessors, but be able to depart from it “at the right time.”
I joined Institut Français du Pétrole in 1960 and managed to focus my work on what I thought would be most interesting. I got married the same year and over the course of time we had two sons and five grandsons.
The oil industry essentially uses heterogeneous catalysis: cracking, reforming, hydrodesulphurization, hydrogenation, etc., but that was not what interested me. I have always tried to avoid areas that have been perfected with time. At the time, nothing much was being done in France on coordination chemistry, organometallics or homogeneous catalysis by transition metals and I was fascinated by the achievements in Italy (G. Natta), Great Britain (J. Chatt), Germany (at the Max-Planck-Institute in Mülheim) and the United States. As a result, I unwittingly became the French specialist in these disciplines, which brought me into contact with both the positive and the unwieldy aspects of the various commissions at the CNRS. I spent the best part of my time on applied chemistry, which was what I had been employed for and which I was quite happy about. This was how I came to develop two homogeneous catalysis processes. The first one, which uses a nickel-based catalyst, was called “Dimersol” and exists in 2 basic versions.
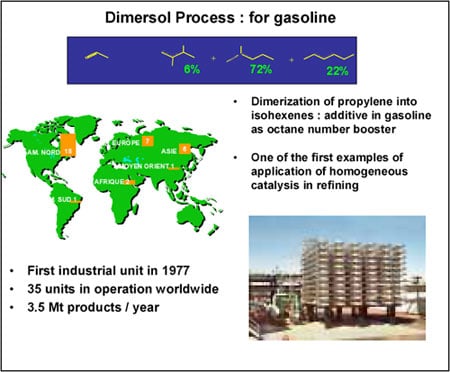
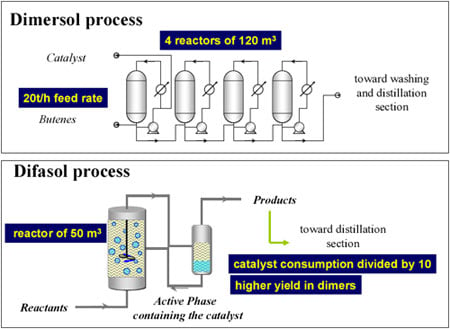
The “gasoline” version (Figure 1) consists of dimerising propene to high-octane isohexenes. There is, quite often, an excess of propene, especially in oil refineries that do not have petrochemicals, as in the United States. There are currently 35 plants in operation (including 18 in the USA ), with a combined annual output of 3.5 million tonnes. It was the first and only time that coordination catalysis had been used in refining.
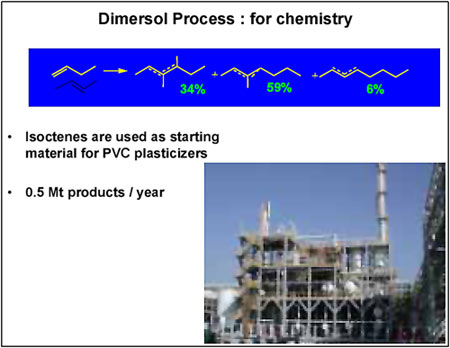
The “chemical” version of the process (Figure 2) consists of dimerising n-butenes to isooctenes, basic inputs for plasticizers, using the “oxo” reaction. Current production levels stand at 400,000 tonnes a year.
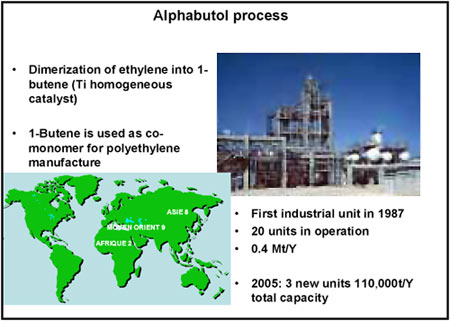
The second process I developed, and which uses a titanium-based catalyst, was called “Alphabutol.” It consists of dimerising ethylene to 1-butene (Figure 3), the co-monomer of low-density linear polyethylene. The benefits of such a process were not evident to begin with and stem from a number of causes. There are currently 20 plants operating worldwide, with a combined output of 400,000 tonnes a year. However, others are under construction, which will take total output to over 0.5 million tonnes a year.
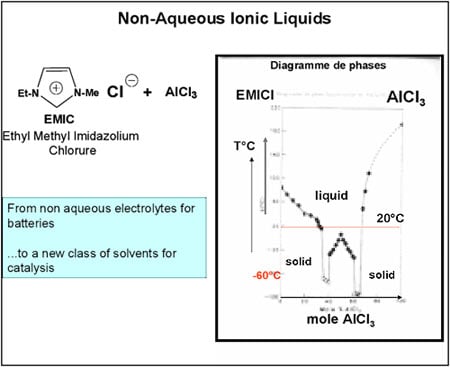
While there are obvious drawbacks to not having done a PhD (especially when you supervise them!), the advantage is that at least your mind is free to focus on whatever presents itself. At the time, I was working on batteries and, in particular, the non-aqueous electrolytes used to extend their electrochemical window. I thought it would be a good idea to try to use these electrolytes, which belong to the class of ionic liquids, as catalyst solvents. These liquids feature very low vapour pressure and virtual non-solubility in hydrocarbons, paving the way for a biphasic catalysis. The mixture of alkylimidazolium chloride and aluminium chloride forms a liquid with a very low melting point (below ambient temperature) (Figure 4). It proved to be a first-rate solvent for nickel-based dimerisation catalysts (“Dimersol” catalysts). The diagram for this process, called “Difasol,” is shown in Figure 5. The reaction volume required for a biphasic system is 10 times smaller than for a homogeneous system (important for safety: refineries do not like to have large volumes in reaction because they are potential “bombs,” especially at start-up); likewise for nickel consumption. This new process, dealt with in a PhD project in 1990, will see the light of day thanks to the inventiveness and determination of Hélène Olivier-Bourbigou, who took over from me in the laboratory.
What applied chemistry has taught me is the need for absolute solidarity between the research laboratory and the “downstream” side (pilot testing, marketing, setting up industrial plant): same enthusiasm, same determination, especially when everything goes wrong!
There is no difference between fundamental research and applied research. Although this is my view, based on personal taste and the areas I have worked in, it is not necessarily true for others. The PhD either led to, or were derived from processes. I have spoken so much about “processes” because they took up about three-quarters of my working time. However, I also took an interest in other aspects of coordination chemistry, such as palladium catalysis, rhodium catalysis, asymmetric amino-acid synthesis, and so on. After retiring in 1995, I was invited to work in J.-M. Basset’s laboratory in Lyon, which allows me to pursue a reasonable level of activity.
This autobiography/biography was written at the time of the award and first published in the book series Les Prix Nobel. It was later edited and republished in Nobel Lectures. To cite this document, always state the source as shown above.
Yves Chauvin died on 27 January, 2015.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
