Illustrated information
Nobel Poster from the Nobel Committee for Chemistry, web adapted by Nobelprize.org
Contents
Great art in a test tube
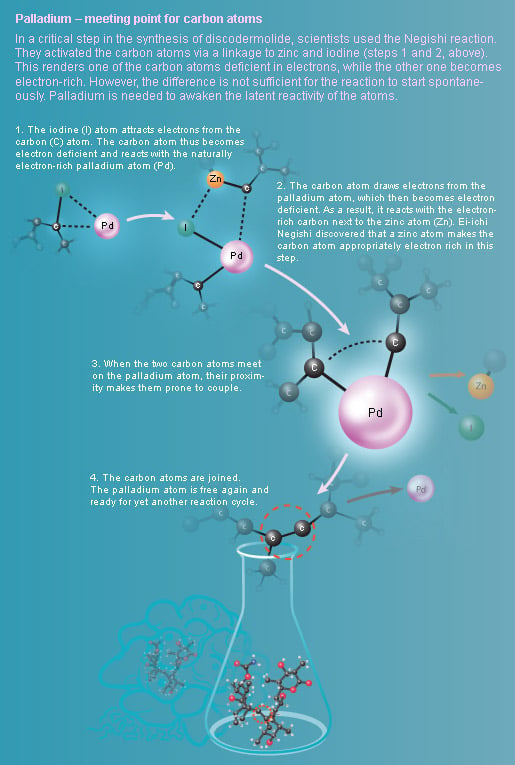
Palladium – meeting point for carbon atoms
The Heck reaction
The Negishi reaction
The Suzuki reaction
Further reading
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry for 2010 to Richard F. Heck, Ei-ichi Negishi and Akira Suzuki, “for palladium-catalyzed cross couplings in organic synthesis“.
Mankind wants more powerful medicines, the electronics industry is searching for lightemitting materials, and the agricultural industry wants substances that can protect crops. The Nobel Prize in Chemistry 2010 rewards a tool that has profoundly improved the ability of chemists to satisfy these wishes: the palladium-catalyzed cross coupling.
Carbon bonds – the skeleton of life
Carbon-based (organic) chemistry is the basis of life and is responsible for numerous fascinating natural phenomena: colour in flowers, snake poison and bacteria killing substances such as penicillin. Through organic chemistry man has learnt how to augment nature’s chemistry, how to build carbonbased functional molecules: drugs, plastics, and other high-tech materials.
Carbon is by nature a stable element and carbon atoms do not easily react with one another. However, in the Heck reaction, the Negishi reaction and the Suzuki reaction, carbon atoms meet on a palladium atom, whereupon their proximity kick-starts the chemical reaction. Palladium-catalyzed cross couplings are of great importance and are used, for example, in approximately a quarter of all reactions performed by the pharmaceutical industry.
The ocean – a large pharmacy
One of many examples where palladium-catalyzed cross couplings have been utilized is in the synthesis of the potentially cytotoxic drug discodermolide. The substance was isolated from the marine sponge Discodermia dissoluta which had been collected by scuba diving marine biologists in the Caribbean Sea at a depth of 33 meters (108 feet). Marine sponges naturally produce complex chemical compounds, toxic molecules that prevent other organisms from exploiting them. These substances sometimes prove to have therapeutic properties. The first laboratory tests on discodermolide revealed that the substance is harmful to cancer cells.

Scientists found the sponge Discodermia dissoluta that contains discodermolide in the Caribbean Sea. Today, they are able to synthesize the complex molecule in a test tube.
But it is impossible to develop a drug from a substance found only in small quantities in the depth of the ocean. To be able to start clinical testing on humans, chemists needed to artificially produce discodermolide in a test tube. When scientists succeeded in building large amounts of the complex molecule for the first time, the Negishi variant of the palladium-catalyzed cross coupling was used.
The Heck reaction
Richard F. Heck began experimenting with using palladium as a catalyst for formation of carbon-carbon bonds at the end of the 1960’s. Among other things, he was able to link a ring of carbon atoms to the carbon of a short molecule, an olefin, in order to obtain styrene, a major component in the plastic polystyrene. In the Heck reaction an olefin is always used as a starting material.
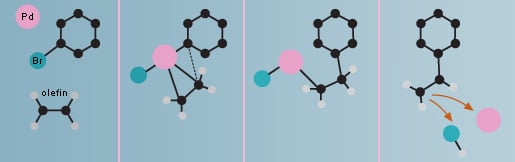
The Heck reaction is used commercially in large-scale production of the anti-inflammatory drug naproxen, the asthma drug montelukast and in a substance used by the electronics industry for the coating of chips.

The Negishi reaction
Ei-ichi Negishi developed another variant of palladium-catalyzed cross coupling in 1977 when he started to use zinc as an activator. The Negishi reaction was for example used when scientists artificially synthesized discodermolide (illustration above).
The Suzuki reaction
In 1979 Akira Suzuki started to use boron in palladium-catalyzed cross coupling. This element is the mildest activator, and it is even less toxic than zinc, which is an advantage in large-scale applications. The Suzuki reaction is for example used in the industrial synthesis (thousands of tons) of a substance that protects crops from fungi. The reaction is also used in research and development; a few examples are shown below.

German scientists are using the Suzuki reaction to create organic polymers that emit light when a current runs through them. The goal is to improve super-thin OL ED (organic light-emitting diode) displays.
Swedish scientists are using the Suzuki reaction to develop n,ew light-capturing molecules. These can be spray-painted onto a surface and could become a part of future flat solar cells.
The Suzuki reaction has been used to develop variants of the antibiotic vancomycin. These variants are effective against strains of bacteria that are otherwise resistant (MRSA).

Richard F. HeckAmerican citizen. Born 1931 in Springfield, MA, USA. Ph.D. 1954 from University of California Los Angeles (UCLA), CA, USA. Willis F. Harrington Professor Emeritus at University of Delaware, Newark, DE, USA. |
Ei-ichi NegishiJapanese citizen. Born 1935 in Changchun, China. Ph.D. 1963 from University of Pennsylvania, Philadelphia, PA, USA. Herbert C. Brown Distinguished Professor of Chemistry at Purdue University, West Lafayette, IN, USA. |
Akira SuzukiJapanese citizen. Born 1930 in Mukawa, Japan. Ph.D. 1959, Distinguished Professor Emeritus, both at Hokkaido University, Sapporo Japan. |
Further reading! |
| Information on the Nobel Prize in Chemistry 2010: http://kva.se/, http://nobelprize.org |
| Review article: |
| Negishi, E. (1999) A profile of Professor Richard F. Heck: Discovery of the Heck reaction, Journal of Organometallic Chemistry 576: XV-XVI. |
| Rouhi, M. (2004) Chem. & Eng. News 82(36):49–58. [Article about Suzuki.] |
| Buchwald, S. L. (red.) (2008) Accounts of Chemical Research, November Issue. [Special issue on Cross Coupling.] |
| Book: |
| de Meijere, A. and Diederich, F. (Eds.), (2004) Metal-Catalyzed Cross-Coupling Reactions, vol. 1 and 2., Wiley-VCH, Weinheim. |
| Links: |
| Chang, K., (2010) 3 Share Nobel in Chemistry for Work on Synthesizing Molecules. The New York Times, www.nytimes.com |
| Coghlan, A., (2010) Chemistry Nobel winner: My work is not done. New Scientist, www.newscientist.com |
Credits and references for the 2010 Nobel Poster for Chemistry
Editors: Jan-Erling Bäckvall, Lars Thelander, The Nobel Committee for Chemistry, Ann Fernholm, Katalys Media, Annika Moberg, Editor and Patrik Engberg, Nobel Assistant, The Royal Swedish Academy of Sciences
Illustrations: Airi Iliste
Layout: Typoform
Print: Åtta.45 Tryckeri AB
Copyright © The Royal Swedish Academy of Sciences
Box 50005, SE-104 05 Stockholm, Sweden
Phone:+46 8 673 95 00, fax: +46 8 15 56 70
e-mail: info@kva.se, http://kva.se
Posters may be ordered free of charge by e-mail to posters@kva.se, phone or fax.
Web adapted version: Nobelprize.org
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
