Press release

NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
The Nobel Assembly at the Karolinska Institute has today decided to award
the Nobel Prize in Physiology or Medicine for 1987 to
Susumu Tonegawa
for his discovery of “the genetic principle for generation of antibody diversity”.
Summary
Man is surrounded by viruses, bacteria and other microorganisms which constitute a threat to life and health. When these contagious agents enter the body they are recognized and attacked by the immune defence. Important tools in the recognition of this large variety of intruders are the antibodies. They are produced by white blood cells called B lymphocytes. The parts of the microorganisms against which antibodies react are called antigens. The number of different antigens that the body may encounter is enormous. We are dealing with hundreds of millions of substances, all of them with their specific structure. Strangely enough our immune defense have at hand antibodies which can identify all these molecules and start to counter attack – that is, hundreds of millions of different antibodies which are ready in the body already in advance before they have seen the antigen against which they can react!
This fabulous capacity to vary of antibodies is known since a couple of decades. The genetic background allowing this variation has, however, been an unsolved puzzle. The structure of the antibodies is determined by genes but as the human genome only contains about 100 000 genes it seemed unreasonable that they could allow the production of maybe a billion different antibodies.
The man who explained this mystery is the Japanese Scientist Susumu Tonegawa. In a pioneering study published in 1976 Tonegawa could through a series of ingenious experiments show how parts of the genome of the cell (DNA) is redistributed under its differentiation from an embryonic cell to an antibody producing B lymphocyte. During the following two years Tonegawa completely dominated this area of research. He could in increasingly greater detail clarify how those parts of the genome which gives rise to antibody are moved around in order to allow each B lymphocyte to produce its own unique antibody.
Tonegawas discoveries have increased our knowledge about structure of our immune defense. They also open up possibilities to increase the immune response against pathogenic microorganism through vaccination – and also to improve inhibition of unwanted immune reactions.
The antibody, a molecule with many faces
The antibody is a protein where the building stones – amino acids normally form four chains. Two of these chains (polypeptides) are long and identical. The other two are short and are likewise identical. Together the four polypeptide chains form a Y-like symmetric molecule (Figure 1).
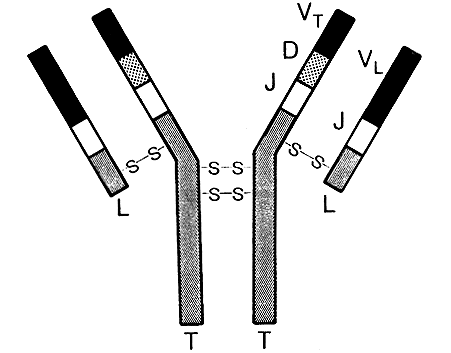 |
Figure 1. A picture of an antibody molecule with two long (T) and two short (L) polypeptide chains which are kept together by sulphur bridges (-S-S). The variable parts of a long chain (V,D, and J) and a light chain (Y and J) together form the antigen binding area of the antibody.
In man there are five different types of long chains which have been given letters M, D, G, A and E. The naming of the long chains forms the bases for the names of the five so called immunoglobulin classes: IgM, IgD, IgG and so on. The short chains are of two types: kappa or lambda. Each antibody molecule has – regardless of class – either two kappa or two lambda chains.
Towards the base of the Y there is a constant part where the sequence of amino acids is the same in all antibodies belonging to the same class. In the outer ends of the two arms of the Y, however, there exist a significant variation in the amino acid sequence when comparing different antibodies. In this variable part there are three areas where variation is very large. These areas constitute the walls in a “pocket” where the foreign substance, the antigen, will fit and can bind. You can make the analogy of an antibody molecule with a lobster where the claws of the lobster correspond to the antigen binding parts of the antibody.
Through its Y-form the antibody accordingly is endow two identical antigen binding areas. These areas have a more or less good fit to a particular antigen. The better the fit the harder to grip of the antigen and the more efficient the defense. As we are continuously confronted within an enormous variety of antigens we also have to have a large number of molecules there the variable parts do fit to different antigens.
The constant part of the antibody does also contain important biological functions. After the binding of the antibody to an antigen on the surface on for example a virus (Figure 2) the antibody molecule is changed in such a way that its constant part will activate important parts of the immune defense. Among these is the complement system which can directly make holes in bacteria and other microorganism and which also attract white blood cells such as macrophages (“big eaters”) and granulocytes to the battle ground.

Figure 2. A polio virus particle is attacked by four IgG antibodies. Through this attack the infectious capacity of the virus is destroyed. It is mainly through this mechanism that polio vaccine is functioning.
The richness of variety, an equation which didn’t add up
Antibodies are produced by a special kind of white blood cells which are called B lymphocytes and which in an adult human being amounts to one million million cells (1012). As a single B lymphocyte only can produce its own unique antibody the number of different antibodies in an individual can theoretically not exceed that of the number of B lymphocytes.
The information how antibodies should be constructed lies in the genome of the B lymphocytes. One hypothesis suggested, that in this genome there exist one gene responsible for each type of polypeptide chain in the antibody. But here the problem was that the immune defense contains hundreds of thousands times more different antibody types than there are total number of genes in the B cells. The equation simply didn’t add up and the hypothesis had to be abandoned. It was replaced by a second one which explained the almost limitless capacity of variation in antibodies as a result of some changes in the DNA of the B cell during the development of the individual.
Susumu Tonegawa was the one who finally answered the question how the gene material in B cells could suffice to create the structures of a seemingly endless number of different antibodies. In 1976 he could in a convincing and elegant manner show how different immunoglobulin genes which were far apart in the embryonic cell in the B lymphocyte had been moved in closer contact. Under development from the germ cells (the sperm and egg cell) to an antibody producing B lymphocyte the genes forming the immunoglobulins had accordingly been redistributed. In subsequent experiment Tonegawa could clarify how different pieces of the genome were moved around, recombined and even could be “lost” to finally give rise to the DNA which is found in the mature B lymphocyte.
In the human the genes for the long chains are present on chromosome 14, for the kappa chains on chromosome 2 and for the lambda chains on chromosome 22. Thanks to Tonegawa’s pioneering work we now know how many immunoglobulin genes there are in man, how they are put together and how they can give rise to this high number of different antibodies.
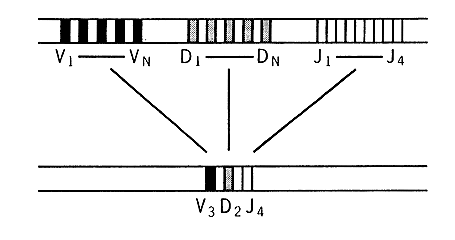 |
Figure 3. Redistribution of immunoglobulin genes for the long chain during the development from an embryonic cell (top) to an antibody producing B lymphocyte (bottom). Genes from each group V, D and J are brought together in the final form for functioning gene for the variable part of the long chain of an antibody molecule.
Economy through waste
Today we know that three groups of genes participate in the creation of the variable part of the long chain, that is the part which together with the variable part of the short chain is specific for each antibody. These genes have the names V, D and J (Figure 3). The short chain has V and J genes. In man the number of different Y genes for the long chains are around 200 to which should be added about 20 D genes and 4 J genes. When the functioning gene of an antibody is to be created a single V, D and J gene are drawn at random from the three groups of genes. The process can be compared to a numbers lottery (Figure 4). 200 x 20 x 4 will give rise to 16 000 different variable parts.

Figure 4. A registration sign for a car with its unique registration number produced through lottery can illustrate the process which leads to the creation of a unique antibody molecule. This registration number stands for Susumu Tonegawa, the 144th Nobel Laureate in Physiology or Medicine.
V, D and J are put together in an irregular manner which will further enhance the richness of variation. And as the V and D genes often are different when inherited from our father and mother this will mean that already here possibility has been created for something like five million different forms of the variable part of the long chain. On top of this the light chain contributes with more than 10 000 variants. The final sum will be many billions possibilities of variation.
We are accordingly well prepared for an encounter with any possible antigen. It is likely that normally only a minor part of the antibody variance will ever be put into usage. The immune system is extremely economic when using the DNA of the individual. At the same time a large number of lymphocytes are produced and only a few of these will ever have to participate in the immune defense of the body. The economy in the usage of DNA is thus combined with a seeming waste of cells. This is, however, necessary to maintain the high state of alertness which is required against possible new infections.
The discoveries of Tonegawa explain the genetic background allowing the enormous richness of variation amongst antibodies. Beyond deeper knowledge of the basic structure of the immune system these discoveries will have importance in improving immunological therapy of different kinds, such as for instance the enforcement of vaccinations and inhibition of reactions during transplantation. Another area of importance are those diseases where the immune defense of the individual now attack the bodies own tissues, the so called autoimmune diseases.
References
P. Leder: The Genetics of Antibody Diversity. Scientific American 1982, 246, 72-83.
E. Norrby: Våra virus. Liber, Stockholm, 1987.
S. Tonegawa: Somatic Generation of Antibody Diversity. Nature 1983, 302, 575-581.
H. Wigzell: Vårt fantastiska immunförsvar. Liber, Stockholm, 1987.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
