Press release
English
Swedish
French
German

NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
11 October 1999
The Nobel Assembly at Karolinska Institutet has today decided to award
the Nobel Prize in Physiology or Medicine for 1999 to
Günter Blobel
for the discovery that “proteins have intrinsic signals that govern their transport and localization in the cell”
Summary
A large number of proteins carrying out essential functions are constantly being made within our cells. These proteins have to be transported either out of the cell, or to the different compartments – the organelles – within the cell. How are newly made proteins transported across the membrane surrounding the organelles, and how are they directed to their correct location?
These questions have been answered through the work of this year’s Nobel Laureate in Physiology or Medicine, Dr Günter Blobel, a cell and molecular biologist at the Rockefeller University in New York. Already at the beginning of the 1970s he discovered that newly synthesized proteins have an intrinsic signal that is essential for governing them to and across the membrane of the endoplasmic reticulum, one of the cell’s organelles. During the next twenty years Blobel characterized in detail the molecular mechanisms underlying these processes. He also showed that similar “address tags”, or “zip codes”, direct proteins to other intracellular organelles.
The principles discovered and described by Günter Blobel turned out to be universal, operating similarly in yeast, plant, and animal cells. A number of human hereditary diseases are caused by errors in these signals and transport mechanisms. Blobel’s research has also contributed to the development of a more effective use of cells as “protein factories” for the production of important drugs.
Several important functions
An adult human being is made up of approximately 100,000 billion cells. A cell contains many different compartments, organelles, each surrounded by a membrane. The organelles are specialized to carry out different tasks. The cell nucleus, for instance, contains the genetic material (DNA) and thus governs all functions of the cell. The mitochondria are the “power plants” producing energy needed by the cell, and the endoplasmic reticulum is, together with the ribosomes, responsible for synthesizing proteins.
Every cell contains approximately one billion protein molecules. The different proteins have a large number of important functions. Some constitute the building blocks for constructing the cell while others function as enzymes catalyzing thousands of specific chemical reactions. The proteins within a cell are constantly degraded and resynthesized. The number of amino acids – the building blocks making up all proteins – may in a single protein range from about 50 to several thousands, forming long, folded chains.
How do proteins cross the barriers?
Thus, it was for a long time a puzzle how large proteins could traverse the tightly sealed, lipid-containing, membranes surrounding the organelles. Some decades ago, it was also unknown how newly made proteins were directed to their correct locations in the cell.
Günter Blobel was going to solve both of these puzzles. At the end of the 1960s he joined the famous cell biology laboratory of George Palade at the Rockefeller Institute in New York. Here, during two decades, scientists had studied the structure of the cell and the principles for the transport of newly synthesized proteins out of the cell. This work earned George Palade the Nobel Prize in Physiology or Medicine in 1974 (which he shared with the Belgian scientists Albert Claude and Christian de Duve).
“The signal hypothesis”
Günter Blobel’s research was built on the traditions of Palade´s laboratory. In particular, Blobel studied how a newly made protein, destined to become transported out of the cell, is targeted to a specialized intracellular membrane system, the endoplasmic reticulum. In 1971 he formulated a first version of the “signal hypothesis”. He postulated that proteins secreted out of the cell contain an intrinsic signal that governs them to and across membranes.
Based on elegant biochemical experiments, Blobel described in 1975 the various steps in these processes. The signal consists of a peptide, i.e. a sequence of amino acids in a particular order that form an integral part of the protein. He also suggested that the protein traverses the membrane of the endoplasmic reticulum through a channel (Fig. 1). During the next twenty years, Blobel and coworkers step by step characterized the molecular details of these processes. Eventually it was shown that the signal hypothesis was both correct and universal, since the processes operate in the same way in yeast, plant, and animal cells.
“Address tags” for organelle localization
In collaboration with other research groups, Günter Blobel was soon able to show that similar intrinsic signals target the transport of proteins also to other intracellular organelles. On the basis of his results, Günter Blobel formulated in 1980 general principles for the sorting and targeting of proteins to particular cell compartments. Each protein carries in its structure the information needed to specify its proper location in the cell. Specific amino acid sequences (topogenic signals) determine whether a protein will pass through a membrane into a particular organelle, become integrated into the membrane, or be exported out of the cell.
A range of signals that govern proteins to the different parts of the cell have now been identified (Fig. 2), showing that the principles formulated by Blobel are correct. These signals can be compared to address tags or zip codes which ensure that a traveler’s luggage arrives at the correct destination, or a letter reaches its correct addressee. These signal sequences are in fact a chain of different amino acids present either as a short “tail” at one end of the protein, or sometimes located within the protein.
Significance of Blobel’s discovery
Günter Blobel’s discovery has had an immense impact on modern cell biological research. When a cell divides, large amounts of proteins are being made and new organelles are formed. If the cell is to function correctly, the proteins have to be targeted to their proper locations. Blobel´s research has substantially increased our understanding of the molecular mechanisms governing these processes. Furthermore, knowledge about the topogenic signals has increased our understanding of many medically important mechanisms. For example, our immune system uses topogenic signals, e.g. in the production of antibodies.
Blobel’s research has helped explain the molecular mechanisms behind several genetic diseases. If a sorting signal in a protein is changed, the protein could end up in a wrong location in the cell. One example is the hereditary disease primary hyperoxaluria, which causes kidney stones already at an early age. In some forms of familial hypercholesterolemia, a very high level of cholesterol in the blood is due to deficient transport signals. Other hereditary diseases, e.g. cystic fibrosis, are caused by the fact that proteins do not reach their proper destination.
Future applications
In the near future the entire human genome will be mapped. As a result one can also deduce the structure and topogenic signals of the proteins. This knowledge will increase our understanding of processes leading to disease and can be used to develop new therapeutic strategies. Already today drugs are produced in the form of proteins, e.g. insulin, growth hormone, erythropoetin and interferon. Usually bacteria are used for the production of the drug, but in order to be functional certain human proteins need to be synthesized in more complex cells, such as yeast cells. With the help of gene technology the genes of the desired proteins are provided with sequences coding for transport signals. The cells with the modified genes can then be efficiently used as protein factories.
Increased knowledge about the process by which proteins are being directed to different parts of the cell also makes it possible to construct new drugs that are targeted to a particular organelle to correct a specific defect. The ability to reprogram cells in a specific way will also be important for future cell and gene therapy.
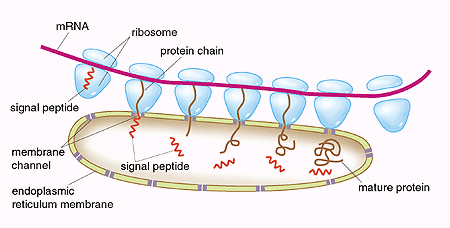 |
| Fig. 1. “The signal hypothesis”. Proteins which are to be exported out of the cell are synthesized by ribosomes, associated with the endoplasmic reticulum. The genetic information from DNA is transferred via messenger RNA (mRNA). This information determines how the amino acids build up the proteins. First, a signal peptide is formed as a part of the protein. With the help of binding proteins, the signal peptide directs the ribosome to a channel in the endoplasmic reticulum. The growing protein chain penetrates the channel, the signal peptide is cleaved, and the completed protein is released into the lumen of the endoplasmic reticulum. The protein is subsequently transported out of the cell. |
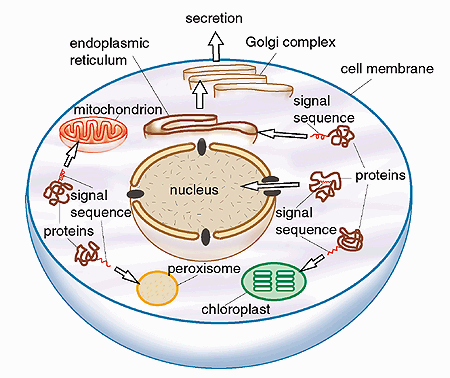
Fig. 2. Examples of directed transport mediated by topogenic signals. The figure shows a schematic cell with some of its compartments, the organelles. (A chloroplast is an organelle that is present in plant cells but not in animal cells). The organelles have special functions and they are surrounded by membranes. Newly synthesized proteins are provided with special “address tags”, signal sequences or topogenic signals, which direct the proteins to a correct place within the cell and allow them to cross the membranes of the organelles. The signal itself consists of a chain of amino acids. It is an integral part of the protein, and it is often located at one end of the protein.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
