Popular information
Information for the public (pdf)
Populärvetenskaplig information (pdf)

The Nobel Prize in Physiology or Medicine 2009
is awarded to
Elizabeth H. Blackburn, Carol W. Greider and Jack W. Szostak
“for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase”
This year’s Nobel Prize is awarded to three scientists who have solved one of biology’s great mysteries: How are the chromosomes that carry our genes copied in their entirety during cell division and protected against breakdown? The Nobel Laureates have shown that the answer lies in the chromosomes’ ends – the telomeres – and in the enzyme that forms them – telomerase.
Elizabeth Blackburn and Jack Szostak were the first to show that the unique DNA sequence contained in the telomeres serves to protect the chromosomes. Carol Greider and Elizabeth Blackburn were then able to show how the telomeres could be extended by the enzyme telomerase.
These discoveries have helped us understand how our genes and cells are maintained throughout life. Telomeres and telomerase also play an important role in various diseases such as cancer. This knowledge has also given us greater insight into how our cells and bodies age.
Sticky chromosomes
Interest in telomeres was first kindled in the 1930s when the researchers Barbara McClintock and Hermann Muller noticed that the ends of chromosome appeared to play some protective role. Barbara McClintock observed that chromosomes lacking end parts became “sticky” – they often fused to each other or got broken down. Similar observations were reported by Hermann Muller, who coined the term telomere from the Greek telos (end) and meros (part).
At that time, the internal structure of the chromosome was essentially unknown. Researchers knew that chromosomes carry the genetic material, and suspected that the telomeres protect it from degradation, but they could not understand how.
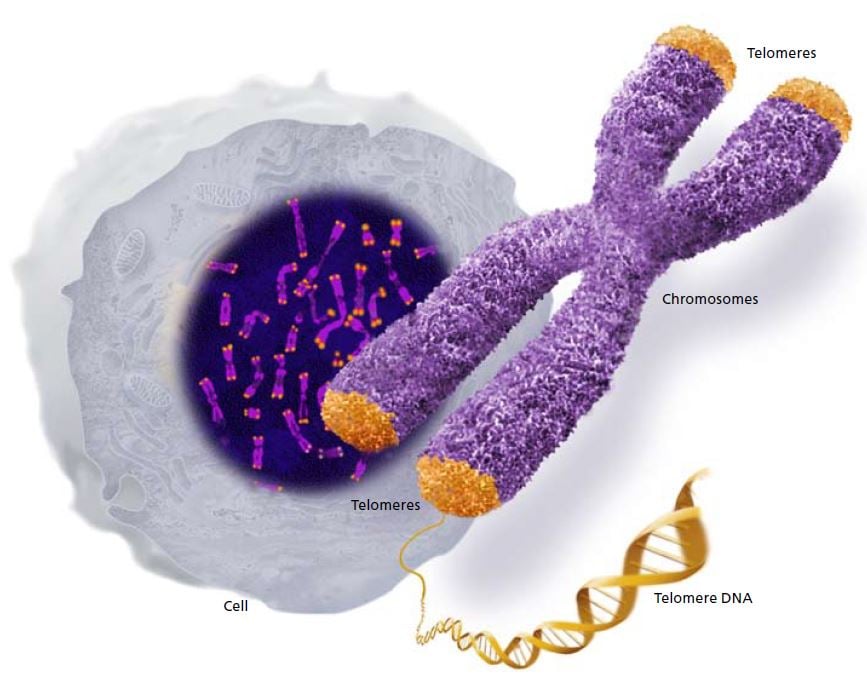
Chromosomes contain the long strands of DNA that comprise our genes. Telomeres form caps at the ends of the chromosomes. © The Nobel Committee for Physiology or Medicine 2009
DNA ends – endless shortening
The beginning of the 1970s saw a new generation of scientists with a molecular focus. Now it was their turn to be puzzled by the ends of chromosomes. James Watson, who had resolved the structure of the DNA molecule along with Francis Crick, pointed out a perplexing circumstance. The established theory of how DNA was copied before cell division could not be reconciled with the fact that DNA strands have ends.
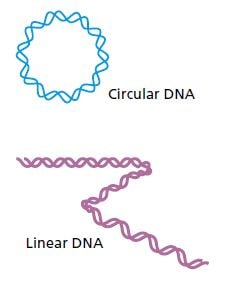
Many primitive organisms such as bacteria have circular DNA molecules which have no ends that need to be copied. Animals and humans, on the other hand, have linear DNA molecules. Try as they might, the researchers could not figure out how the DNA strands could be copied without losing bits off their ends. After repeated replication and shortening, the genes and cells would be damaged. The question was how cells manage to escape DNA truncation. This quandary, known as the endreplication problem, was elegantly resolved by the discoveries for which this year’s Nobel Prize is awarded.
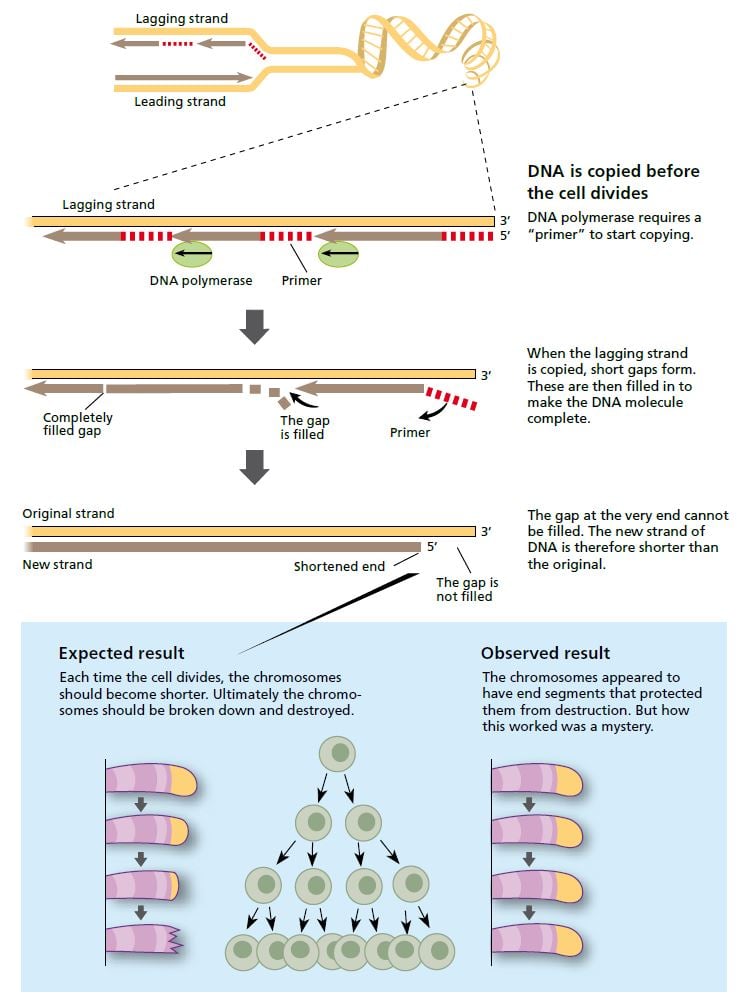
When a cell divides, the strands of the DNA molecule in the chromosome separate and are copied by the enzyme DNA polymerase. One strand (the leading strand) is copied in all in one go. The other (lagging) strand is copied in short segments. © The Nobel Committee for Physiology or Medicine 2009
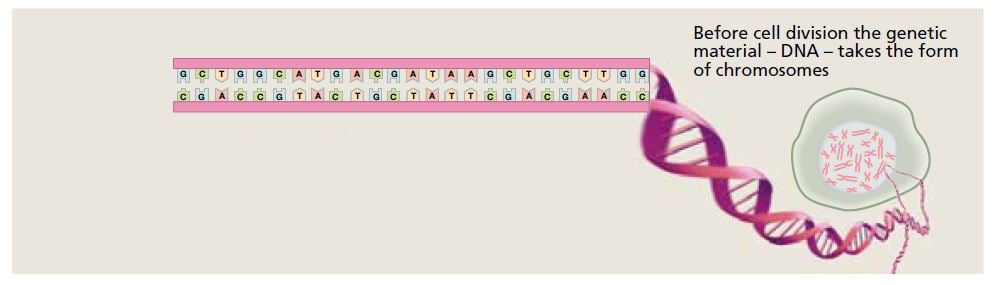
The DNA molecule contains the bases adenine (A), guanine (G), cytosine (C) and thymine (T). Their sequence defines the genetic code. © The Nobel Committee for Physiology or Medicine 2009
A unique DNA sequence
Elizabeth Blackburn did her doctoral research at Frederick Sanger’s laboratory in Cambridge, England, where a completely new technique for determining DNA sequences had been developed. When she went to Yale University in the United States in 1975 as a fresh post doc, she wanted to use her new knowledge to probe the composition of the genes. But at the time, it was extremely difficult to get hold of large enough amounts of genetic material to do that type of analysis.
Her new mentor Joseph Gall had shown that the single-celled organism Tetrahymena contains a multitude of relatively short linear DNA sequences (minichromosomes), which made it possible to isolate large amounts of this specific type of genetic material. Elizabeth Blackburn began to analyze the ends of the minichromosomes, the telomeres, and found a short DNA sequence, CCCCAA, which was repeated twenty to seventy times.
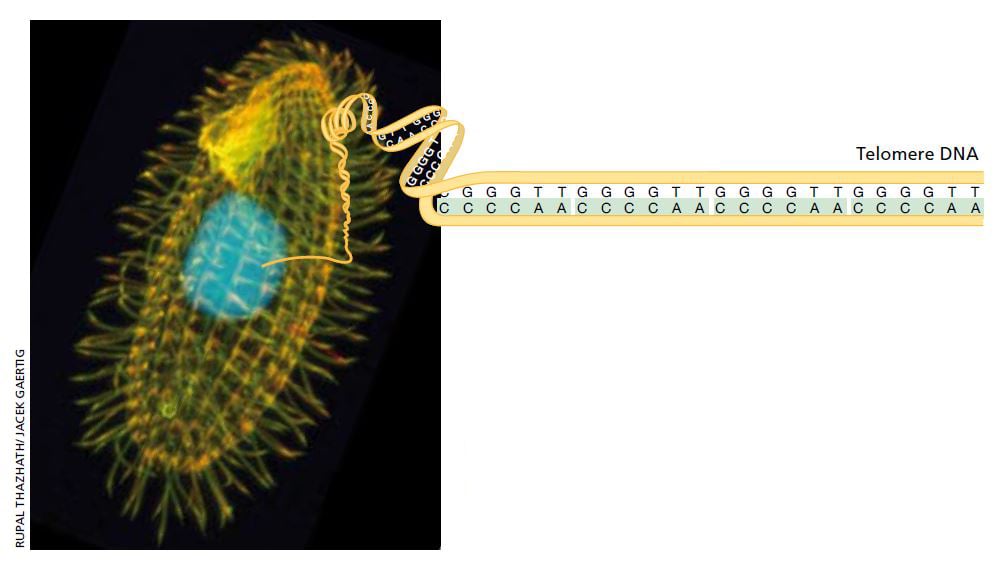
Tetrahymena is a single cell freshwater ciliate. Its peculiar internal structure has attracted scientists’ interest. Each individual Tetrahymena contains over 40,000 telomeres, making it the perfect starting point for examination of telomere DNA. Image: Rupal Thazhath/ Jacek Gaertig. Illustration: © The Nobel Committee for Physiology or Medicine 2009
That was an interesting observation, but the function of this DNA sequence was still completely obscure and the findings raised more questions than they gave answers. It was particularly difficult to explain why the number of sequence repeats varied from one minichromosome to another. It appeared that this DNA sequence was not replicated in the usual way, but was added to the ends of the chromosomes afterwards. But how?
The breakthrough – a tale of two species
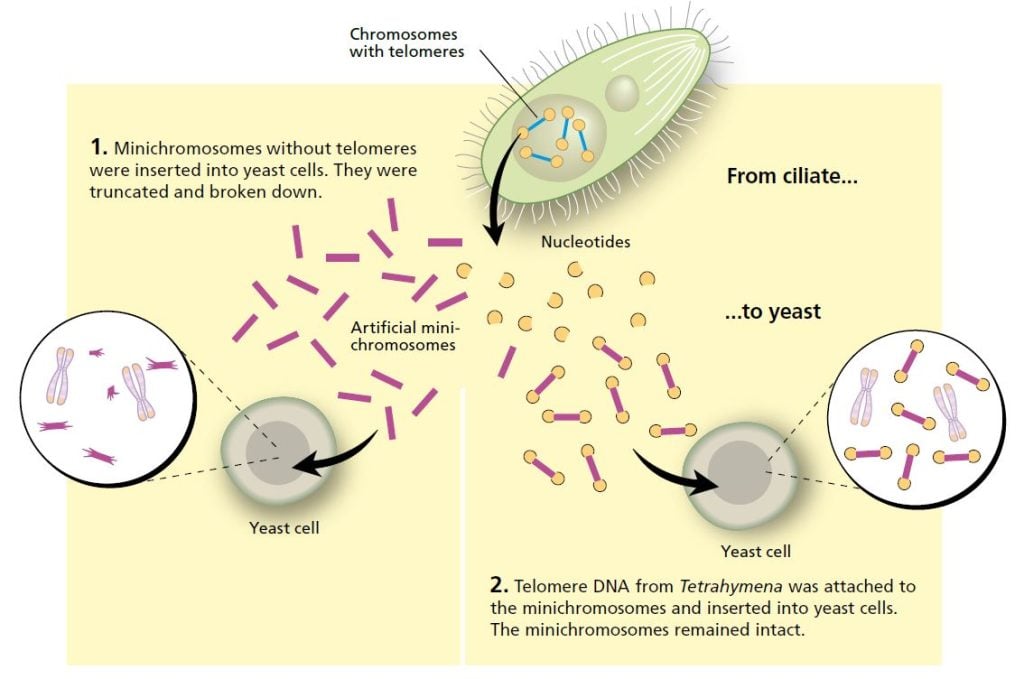
Blackburn described her intriguing findings about Tetrahymena at a scientific conference in 1980. Among the listeners was Jack Szostak, a researcher from Harvard Medical School in the United States, who had a problem on his hands. He wanted to find out how artificial linear DNA sequences, minichromosomes, functioned in yeast, but was impeded in this work because the DNA he inserted into yeast cells quickly broke down.
Szostak realized that he and Blackburn were working with similar issues. Even though the organisms they were studying were highly dissimilar, he suggested an experiment that would transcend the boundary between species. Could the telomere sequences from Tetrahymena protect minichromosomes from being broken down in yeast? This was a bold hypothesis, but the experiment was easy to do and the question was interesting. The two researchers decided it was worth a try.
Blackburn, who was now head of her own laboratory at the University of California, Berkeley, purified the telomere sequences from Tetrahymena. Szostak linked them to minichromosomes and inserted them into yeast cells. The results, published in 1982, were striking: the telomere DNA sequence protected the minichromosomes from degradation.
It was now clear that it was the unique DNA sequence that gave the telomere its protective properties. The fact that telomere DNA from one organism, the single-celled ciliate Tetrahymena, could protect DNA in a completely different organism, yeast, indicated that this was a fundamental mechanism, common for several widely disparate species.
The sequence crops up elsewhere
Szostak refined the experiment and was soon able to identify the yeast’s own telomere sequence. He did this by removing one of the telomeres from the artificial chromosomes that he and Blackburn had created, and replacing it with different DNA fragments from yeast cells. Most of the chromosomes were quickly degraded. But eventually he found a DNA sequence that could stabilize the chromosomes in a yeast cell.
It turned out that telomeres from yeast cells also contained a short repeated DNA sequence, one very similar to that found in Tetrahymena. Later research has shown that this is true of telomeres from most plants and animals – from amoeba to human. We now know that the repeated sequence attracts and binds specific proteins that form a protective cap around the fragile ends of the DNA. Without this cap, the DNA ends tend to stick to each other – the chromosomes fuse together.
The ends change length!
When Blackburn and Szostak analyzed chromosomes that had been in yeast cells for some time, they discovered that the telomeres had grown longer. Several studies confirmed that telomeres can both grow and shrink. Just as James Watson predicted, DNA molecules are truncated during cell division, but they also seem to grow longer through addition of DNA sequences.
This puzzled the two researchers. Enzymes that make DNA were nothing new. These DNA polymerases can “read” and make an exact copy of an existing DNA sequence. But now the task was not to copy from a DNA template, but rather to add an extension to a DNA sequence. Could there be an unknown enzyme that constructs DNA in this way?
The discovery of telomerase
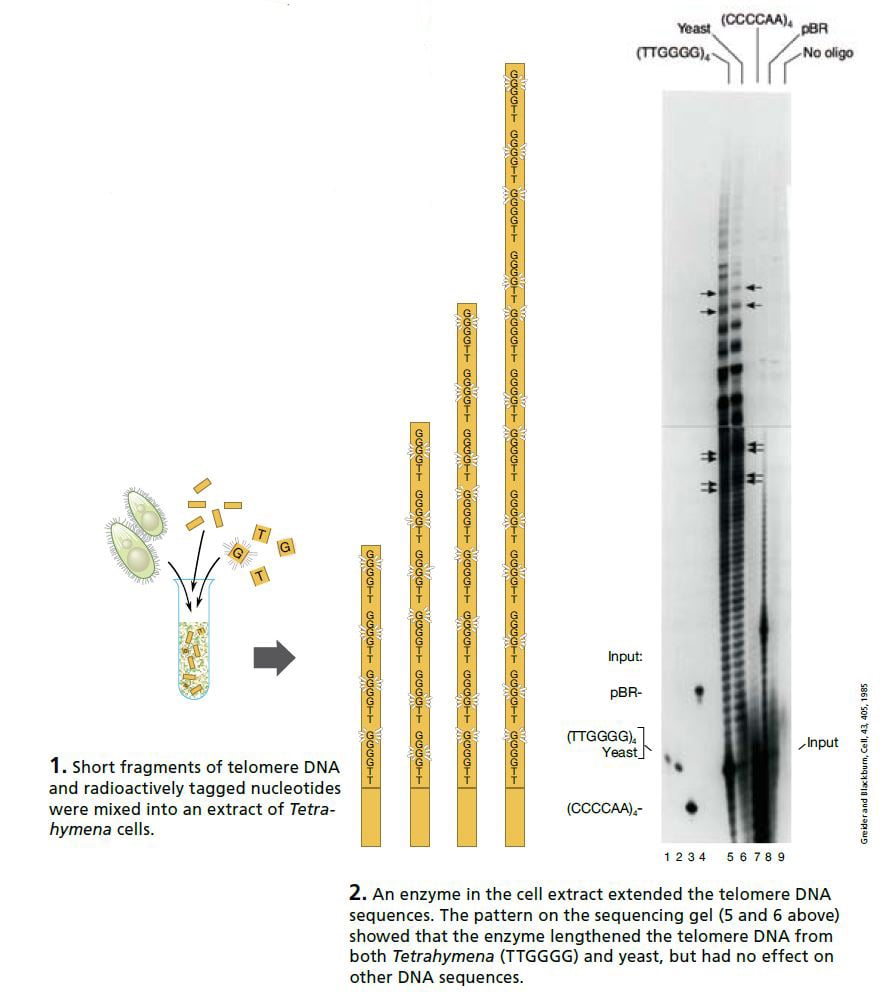
Carol Greider, a 23-year-old doctoral student working in Blackburn’s laboratory at Berkeley, took on this interesting challenge. Greider and Blackburn made an extract of Tetrahymena cells and added a mixture of short telomere DNA sequences and radioactively tagged nucleotides (the building blocks of DNA). If the extract contained only the usual DNA polymerases, nothing should happen. But if the extract contained the enzyme they were looking for the telomeres should grow, incorporating the radioactively tagged nucleotides.
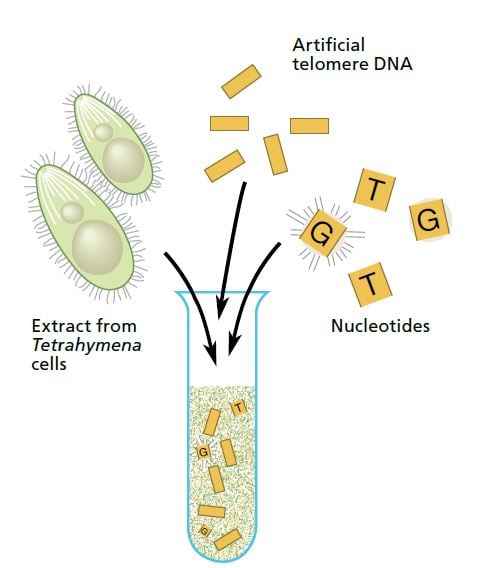
On Christmas Day 1984 Greider saw the first proof of this new enzyme activity. The sequencing gel showed a neat ladder pattern.
DNA fragments that had been identical when they were mixed into the extract were now of different lengths: the radioactively tagged nucleotides had been added – and in exactly the order characteristic of Tetrahymena telomeres. An entirely new enzyme had been discovered. Shortly after, it was named “telomerase”.
The discovery of telomerase
A construction worker with a built-in blueprint
In the years following this discovery, Blackburn and Greider tried to figure out how telomerase could accomplish its task. The key question was easy to pose but difficult to answer. How did the enzyme in the cell extract “know” the order in which the nucleotides should be added?
After several years’ work Blackburn and Greider presented the model that is now generally accepted. Just like other enzymes, telomerase has a protein domain that can synthesize DNA sequences. But telomerase is unique in that it also possesses a segment of RNA (closely related to DNA) which has the same sequence as one of the DNA segments in the telomere. This means that telomerase carries a built-in blueprint for the telomere it constructs.
In 1989, Greider and Blackburn were able to demonstrate that the RNA component of telomerase actually does contain a sequence that corresponds to that of the telomere. The ultimate proof that the model must be correct came from Blackburn’s group one year later. The researchers inserted mutations in the telomerase’s RNA sequence and showed corresponding changes in the DNA of the resulting telomeres.
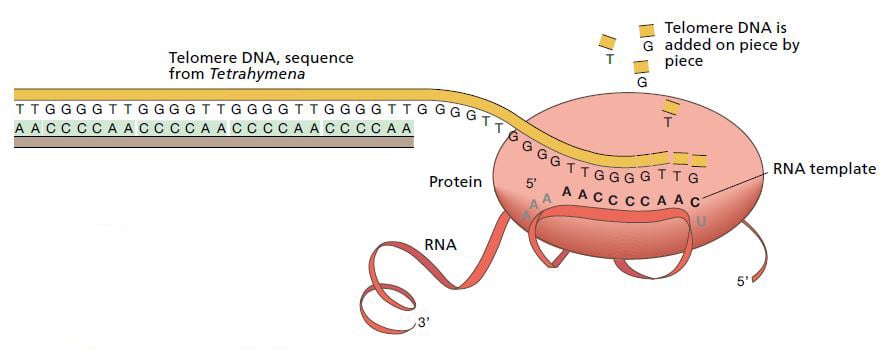
The enzyme telomerase extends the DNA sequence at the ends of chromosomes. It consists of a protein and an RNA sequence. The RNA part functions as a template for the construction of new telomere DNA. © The Nobel Committee for Physiology or Medicine 2009
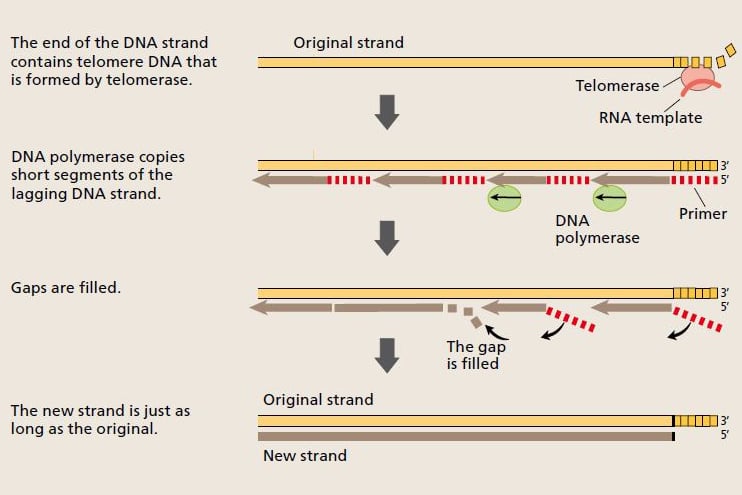
Now the scientists understood how the cell was able to pass on full-length chromosomes to the daughter cells during cell replication. Telomerase extends the telomere, and the telomere DNA provides a platform that enables DNA polymerase to copy the entire length of the chromosome. © The Nobel Committee for Physiology or Medicine 2009
Shrinking telomeres and ageing cells
Now it was time to start dissecting the telomere’s role in the life of a cell. Szostak’s group identified mutations in yeast cells that made telomeres gradually shorter. Cell growth tapered off and the cells eventually stopped dividing. Blackburn and her colleagues inserted mutations into Tetrahymena telomerase RNA and observed similar effects. In both cases, the changes made cells age prematurely, a phenomenon called senescence. They concluded that an intact telomere protects the chromosome from damage and postpones the cell’s ageing.
These basic mechanisms were discovered in single-celled organisms, but in 1988 a similar DNA sequence was identified in human telomeres. This paved the way for studies of the telomere’s role in the human body.
A clock that measures out ageing?
In the 1970s, the Russian scientist Alexei Olovnikov made an interesting association. Perhaps the end-replication problem explained why human cells could only divide a limited number of times? Skin cells from a newborn baby can divide 80 to 90 times in culture, whereas cells from a 70-year-old can only divide 20 to 30 times. Olovnikov suggested that the chromosomes were truncated every time the cell divides, and that the reason cell replication eventually stops is that chromosome length has dropped below a critical limit.
Sure enough, Greider’s group could show that telomeres in human cells are truncated during cell division. The results roused excitement in the research community and some speculated that telomere shortening could be what causes ageing not just of the cell, but of the entire organism. Maybe the telomere serves as a tallying device that keeps track of how long the cell can continue to divide?
Although this notion is attractive, we now know that it gives an incomplete picture of ageing. For example, we have realized that even if most cells have limited capacity to replicate in culture, cells in our bodies can probably divide more times than they will ever need to during a human life-span. Research on ageing has advanced on many fronts and now we know that ageing is influenced by a multitude of factors, telomere function being one of them. The role of telomeres in ageing continues to be the focus of intense research.
Telomerase and cancer
Most normal cells seldom divide. Their chromosomes do not become too short and their telomerase does not have to be particularly active. Cancer cells, on the other hand, have an unlimited capacity to divide. But how can they retain their telomeres and escape senescence?
It has been shown that 80 to 90 percent of all cancer cells have abnormally high telomerase activity. This prevents them from losing their telomeres despite going through many cell replication cycles. Scientists believe that successive telomere shortening in normal cells can be an important protective mechanism to counteract the uncontrolled cell division that characterizes cancer.
This has aroused hope that it will be possible to treat cancer by blocking telomerase, using either substances that inhibit telomerase activity, or vaccines that cause the immune system to to attack cells with excessive telomerase activity. Several such vaccines are currently being tested in clinical trials on humans.
But many problems remain to be overcome before telomerase can be an effective target for cancer therapy. Merely reducing telomerase activity may not be enough, because some cancer cells have found alternative means of producing telomeres, independent of telomerase. There is also a risk of damage to healthy cells in which high telomerase activity is normal, particularly stem cells.
The importance of functional telomeres in the body’s stem cells is illustrated by the fact that several congenital diseases arise because of defective telomerase. These include some severe forms of inherited anemia, where inadequate replication of stem cells in bone marrow leads to a lack of red blood cells. Certain inherited diseases of the skin and lung are also related to telomerase defects.
Guided by curiosity
The discoveries for which this year’s Nobel Prize is awarded are typical examples of curiositydriven basic research. The discoveries were made without any clear medical application, and were initially of interest only to a few scientists within a narrow field. This quickly changed later on, not least as the relevance for human biology began to emerge.
It is still too early to say where the saga of the telomeres will end. So far it is clear that these discoveries not only provide a new picture of the inner working of the cell, but also shed light on important medical principles and suggest new treatment options.
Key publications
Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell 1982; 29:245-55.
Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985; 43:405-13.
Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989; 337:331-7.
The Laureates
Elizabeth H. Blackburn is a citizen of Australia and the United States. She was born in 1948 in Hobart, Tasmania, Australia. She studied at the University of Melbourne and received her PhD in 1975 from the University of Cambridge, England, after which she worked at Yale University in New Haven, USA.
In the 1980s she did research at the University of California, Berkeley, and since 1990 she has been Professor of Biology and Physiology at the University of California, San Francisco.
Carol W. Greider is a United States citizen. She was born in 1961 in San Diego, California, USA and received her education at the University of California in Santa Barbara and Berkeley, where she received her PhD in 1987, with Blackburn as supervisor.
After research work at Cold Spring Harbor Laboratory in New York, she became Professor in the Department of Molecular Biology and Genetics at Johns Hopkins University School of Medicine in Baltimore, in 1997.
Jack W. Szostak is a United States citizen. He was born in 1952 in London, England and grew up in Canada. He studied at McGill University in Montreal and received his PhD from Cornell University in Ithaca, New York in 1977.
Since 1979 he has worked at Harvard Medical School, where he is now Professor of Genetics at Massachusetts General Hospital in Boston. He is also affiliated with the Howard Hughes Medical Institute.
Previous Nobel Prizes in this research field
This year’s award touches on aspects of several earlier Nobel Prizes awarded for research on the genome and how it is composed, copied and distributed during cell division.
1933: Thomas Hunt Morgan “for his discoveries concerning the role played by the chromosome in heredity”.
1946: Hermann Joseph Muller “for the discovery of the production of mutations by means of X-ray irradiation”.
1958: Joshua Lederberg “for his discoveries concerning genetic recombination and the organization of the genetic material of bacteria”.
1959: Severo Ochoa and Arthur Kornberg “for their discovery of the mechanisms in the biological synthesis of ribonucleic acid and deoxyribonucleic acid”.
1962: Francis Crick, James Watson and Maurice Wilkins “for their discoveries concerning the molecular structure of nucleic acids and its significance for informa- tion transfer in living material”.
1975: David Baltimore, Renato Dulbecco and Howard Temin “for their discoveries concerning the interaction between tumour viruses and the genetic material of the cell”.
1983: Barbara McClintock “for her discovery of mobile genetic elements”
2001: Leland Hartwell, Tim Hunt and Paul Nurse “for their discoveries of key regulators of the cell cycle”.
In addition to these Nobel Prizes in Physiology or Medicine, research in this field has been awarded a Nobel Prize in Chemistry.
1980: Walter Gilbert and Frederick Sanger “for their contributions concerning the determination of base sequences in nucleic acids”.
The editorial committee for this year’s popular presentation of the Nobel Prize in Physiology or Medicine included the following, who are professors at Karolinska Institutet and members of the Nobel Assembly:
Göran K. Hansson, Klas Kärre, Nils-Göran Larsson, Thomas Perlmann and RuneToftgård
Text: Ola Danielsson, medical journalist
Translation: Janet Holmén, editor
Illustrations: Bengt Gullbing and Annika Röhl
© 2009 The Nobel Committee for Physiology or Medicine, Karolinska Institutet, 171 77 Stockholm
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
