Advanced information
Scientific background:
Activation of the immune system (pdf)

Scientific background
Activation of the immune system
The 2011 Nobel Prize in Physiology or Medicine is awarded with one half jointly to Bruce A. Beutler and Jules A. Hoffmann for their discoveries concerning the activation of innate immunity, and the other half to Ralph M. Steinman for his discovery of the dendritic cell and its role in adaptive immunity (1). The work of the Nobel Laureates has revolutionized our understanding of the immune system. As a consequence of their discoveries, new fields of research have opened up that hold the promise to improve vaccination and treatment against infection, cancer and inflammatory diseases.
The discoveries
Jules Hoffmann and collaborators used the fruit fly, Drosophila melanogaster, as a model system to study fundamental aspects of how our first line of defense against microbes (innate immunity) is induced. Hoffmann demonstrated that a particular gene, denoted “Toll”, was necessary for fruit flies to fight a fungal infection (2). He concluded that the product of the Toll gene was involved in sensing pathogenic microorganisms and triggering host defense against them. Bruce Beutler and collaborators used a mouse model to search for a gene that activated innate immunity when exposed to a bacterial component (lipopolysaccharide; LPS). Beutler’s work, through the identification of a mutated Toll‐like gene, Tlr4, in LPS resistant mice (3), unraveled the function of Toll‐like receptor (TLR)‐4 as a mammalian counterpart of Drosophila Toll in response to infection. Taken together, these two discoveries uncovered a molecular sensor system (Toll/TLR), shared by insects and mammals, which is essential to induce the first line of defense against microbes.
Ralph Steinman studied the immune system in mice, aiming to understand which cells are important for the activation of the adaptive immune system, i.e., the second line of immune defense where T and B lymphocytes are triggered to mount efficient responses against pathogenic microbes. Steinman discovered a cell with a dendritic appearance in lymphoid organs of mice (4), and in subsequent studies showed that this cell had an exceptional ability to activate T lymphocytes (5). He termed the cell the “dendritic cell” (DC). Through a series of ingenious experiments performed over several years, Steinman discovered a functional system in which maturation of DCs represents a crucial process in enabling the activation of T cell responses (6). Work by Steinman and other scientists has shown that signals mediated by molecules of the innate immune system, including the TLRs, induce the maturation of DC, thus providing a link between innate immunity and adaptive immunity.
Earlier research and seminal discoveries in immunology
Humans as well as all other species are dependent on efficient defense systems against invading microorganisms for their survival. Research on the immune system has consequently been of great importance for our understanding of how we can defend ourselves against microorganisms to survive their threat. This research has also led to novel diagnostics and therapies.
A number of discoveries within the field of immunology have been awarded the Nobel Prize in Physiology or Medicine (1). The very first Nobel Prize, in 1901, was given to von Behring for his studies of protection against Diphtheria by antibody transfer (at the time called serum therapy). The 1908 Nobel Prize to Ehrlich and Mechnikov was the first to recognize the existence of two different arms of the immune system, i.e., a first line of “cellular” defense that involves macrophages (Mechnikov) and a second line of “humoral” defense that involves antibodies (Ehrlich). It was later realized that there are cellular and humoral factors involved both in the first line and in the second line of defense, and the terms “innate immunity” and “adaptive immunity” for these two levels gradually emerged. That the immune system has a capacity to tolerate self and strike efficiently against non‐self through adaptive immune reactions was the subject of another Nobel Prize (Burnet and Medawar 1960). Genes and molecules of the major histocompatibility complex (MHC) that control immune and transplant reactions were discovered by Benacerraf, Dausset and Snell (Nobel Prize 1980). The same MHC molecules were subsequently found to direct the adaptive immune defense against microbes such as viruses (Doherty and Zinkernagel; Nobel Prize 1996). Further discoveries on the structure and generation of antibodies have taught us how these molecules can be generated with an enormous diversity, and how they can recognize and kill myriads of extracellular microorganisms (Nobel Prize to Edelman and Porter 1972, and to Tonegawa 1987). The clonal properties of adaptive immune responses made possible the generation of monoclonal antibodies (Jerne, Köhler and Milstein; Nobel Prize 1984), which opened the door for a whole new generation of diagnostics and therapies.
But despite this progress, many features of the immune system have remained poorly understood. Fundamental problems that have been addressed by this year’s Nobel Laureates are the activation of innate immunity and the role of accessory cells in the activation of adaptive immunity.
Previous progress and challenges concerning the innate immune system
A first line of the immune defense, also called the innate, natural or “inborn” immune system, is important for survival in all organisms that can be attacked by microorganisms in their environment. Although some components of the innate immune system, including several cells as well as soluble molecules, had been characterized before, there was until the mid‐1990ies a crucial lack of knowledge regarding the activation of this system. In particular, it was unclear how its cells sense and become activated by microbial components. A prevailing paradigm was that cells of the innate immune system are activated without any specificity or stimulating triggers. Indeed, this defense was often referred to as “non‐specific” immunity.
Several lines of research led to the discovery of receptors that trigger activation of innate immunity upon microbial recognition. One line of research took off in the 1980’s when several scientists investigated how the defense against microorganisms is accomplished in evolutionarily older organisms that have not developed any second (adaptive) line of immunity. An important step in this process came from the recognition that insects, including fruit flies, produce antimicrobial peptides that are involved in the protection against pathogenic microorganisms. The late Hans G. Boman and his colleagues were particularly active in this field; they unraveled an inducible antibacterial defense in Drosophila (7) and discovered the cecropins (8), now known as one among several classes of soluble antimicrobial peptides that can bind to and lyse bacteria. Today, we know that such peptides comprise an essential part of the innate immune defense, not only in insects but also in mammals, including humans (9). A number of scientists made important discoveries relating to other effector molecules in innate immunity and the genetic elements that regulate their production in insects (10,11), but it remained unclear how these responses were induced by microbes or microbial products. As to mammals, the prevailing view was that the specific triggering stimuli in immune responses exclusively related to adaptive immunity. However, it was known from many studies that a number of different, more or less well defined, stimuli including bacterial substances could enhance the effects of vaccines, likely via activation of cells of the innate immune system. This, and other insights, led the late Charles A. Janeway to postulate the existence of a recognition system for microbial components (“pattern recognition receptors”) (12), different from the antigen specific receptors of T and B lymphocytes. However, there were no clues regarding the molecular nature of the sensing mechanisms.
Discovery of receptors that trigger activation of innate immunity
Jules Hoffman and his colleagues studied mechanisms that activate innate immunity in Drosophila. A major tool in this work, and the one that enabled the major breakthrough, was the availability of mutant fruit flies that had originally been developed in Christiane Nüsslein‐Volhard’s laboratory for studies of embryonic development (13). Her work was awarded the Nobel Prize in 1995. One of these previously generated mutants was called Toll; the legend tells that this name was coined by Christiane Nüsslein‐Volhard after seeing a very peculiar type of fruit fly in the microscope and exclaiming “das war toll” (this looks “great” or “peculiar”). The peculiar appearance of this mutant fly was caused by a defect in the dorsoventral patterning during development.
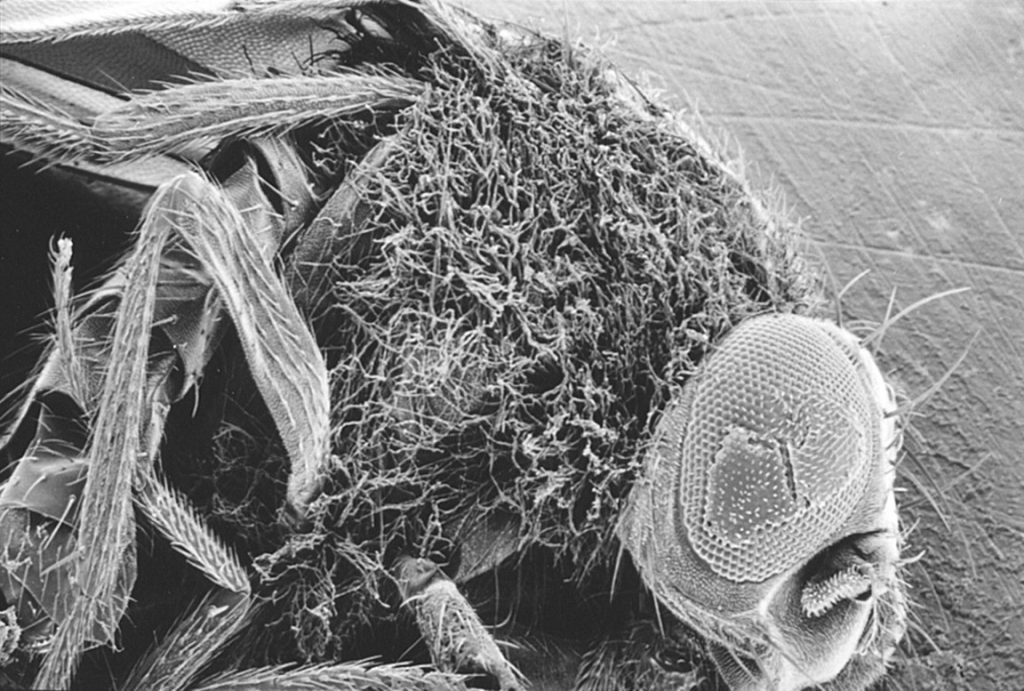
Studies in several laboratories in the early to mid 1990´s, including Hoffmann’s, had suggested similarities between signaling cascades involved in dorsoventral patterning and activation of immune genes in Drosophila (10,11). At the time, this field of research had developed significantly with key contributions by several investigators (14‐25). Findings in this area inspired Hoffmann and collaborators to address the possible role of the Toll gene in sensing infection leading to induction of innate immunity. When Hoffman and his colleagues infected Drosophila that carried a temperature sensitive mutation in the Toll gene with a fungus (Aspergillus fumigatus), all of the flies died due to impaired defense (2). It is now known that the Toll receptor binds another molecule (Spätzle) that in turn recognizes structures present on the fungus and that formation of the Toll/Spätzle complex is essential for activation of innate immunity against fungal infection in the fly (26‐28). A classical picture of a fruit fly with a mutated Toll gene succumbing to a fungal infection (Figure 1) provided the cover of the journal issue in which this paper was published (2). This discovery was a breakthrough, as it identified a specific receptor complex involved in activation of innate immunity upon infection with a pathogenic microorganism.
Were such receptors, with ability to sense microbial components, conserved in mammals? Toll‐like genes had been identified in the human genome 1994‐1997 (29‐32). Some of them had been isolated and characterized as cDNA clones with unknown function (29). Furthermore, in 1997 the gene for a Toll‐like receptor (TLR) called TLR4 had been cloned and engineered to demonstrate that it could activate NF‐kB translocation to the nucleus, a typical immune activation pathway (30). This was an important finding, but it remained unclear whether this receptor had a role in pathogen sensing.
Bruce Beutler solved this question. He had since several years studied the mechanisms involved in the induction of septic shock by Gram‐negative bacteria. This phenomenon had been known since 1892 (33). It involves a systemic response, found later to be elicited by LPS, and can be lethal in an individual exposed to high doses of LPS or bacteria (34). At a low infection dose, the response can instead contribute to protection of the host (33). A most important tool in the research on septic shock was discovered in the 1960s, namely that the C3H/HeJ mouse strain has a severely impaired LPS response (35). The notion of a defective receptor as a mechanism behind the defective LPS response in C3H/HeJ mouse emerged gradually through work by several scientists including Beutler, each providing pieces into a still highly mysterious puzzle. The contributions included the finding that a single locus is responsible for the defect, that the same locus also controls certain B‐cell responses, that macrophages are crucial for the response, and that TNF is a major soluble mediator of the response, as well as identification of other (non signal‐transducing) components of the receptor complex such as CD14 (36‐50).
With these pieces of the puzzle at hand, Beutler launched a long‐term search aimed at identifying the receptor in the mouse that could recognize bacterial LPS and activate the innate immune system (3). He used genetic strategies in a search that would take several years (Figure 2), ending with a discovery that could not have been expected at the initiation of the project.
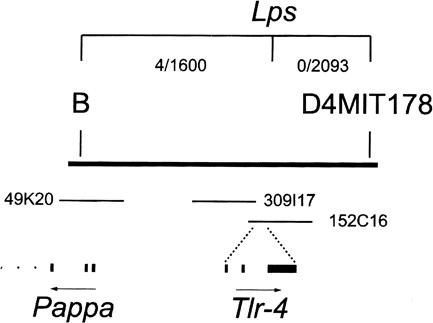
Thus, his team finally identified the signal transducing “LPS receptor”, and revealed by DNA sequencing that it was homologous to the Toll gene in Drosophila (3). The gene identified by Beutler was mutated in the C3H/HeJ strain as well as in another LPS resistant mouse strain. Beutler´s seminal discovery galvanized the field by providing an infection related, non‐redundant, in vivo function for TLR in mammals, as well as the first pathogen derived ligand for a mammalian TLR. His discovery was soon confirmed by others in the field working on similar lines of research (51).
Together, the discoveries of Hoffmann and Beutler demonstrated that very similar receptors are involved in sensing the presence of invading microorganisms and activating innate immunity in a way that ultimately may determine life or death of an animal. Several other scientists were soon able to confirm and extend their findings in other species including man. Taken together, this research has provided us with a completely new view of how “pattern recognition receptors” (12,52), among them the family of Toll‐like receptors, sense and activate innate immunity against different microorganisms. Toll‐like receptors have also been suggested to be involved in recognition of endogenous TLR ligands from within the body, for example after trauma (53).
Following the findings by Hoffmann and Beutler, the field virtually exploded. Ten and twelve TLRs have been identified in humans and mice, respectively, and all are transmembrane proteins with extracellular leucin‐rich repeats and an intracellular Toll/interleukin‐1 receptor (TIR) domain. Some of these are expressed on the outer cell membrane, other in intracellular vesicles. Some receptors recognize bacterial glycoconjugates or proteins, other bind to aberrant nucleic acid patterns typical of bacterial or viral infections (54). Additional receptor families with similar function have been identified. Detailed insights into signal transduction and gene regulation have been obtained, and effector functions have been characterized in large detail. Stimulation of different combinations of receptors has been shown to elicit distinct abilities of DCs to stimulate T lymphocytes and thus generate appropriate responses from the adaptive immune system (55,56).
Previous progress and challenges concerning the adaptive immune system
There has been great progress over the recent decades in our understanding of how B and T cells (lymphocytes) of the adaptive immune system recognize their targets called antigens. When Ralph Steinman entered the field in the early 70´s, it was known that antigens could by themselves not trigger lymphocytes. “Accessory cells” were needed, which could be removed from cell cultures by adherence to plastic surfaces. It was, however, not known whether all of these macrophage‐like cells had the same ability to present antigens to T cells or whether some cells were more important than others. In the prevailing paradigm, the role of the accessory cells was mainly considered in terms of merely delivering the antigen, while T cells were considered to take all the necessary decisions required to orchestrate the ensuing response.
A cell with an exceptional capacity to activate the adaptive immune system
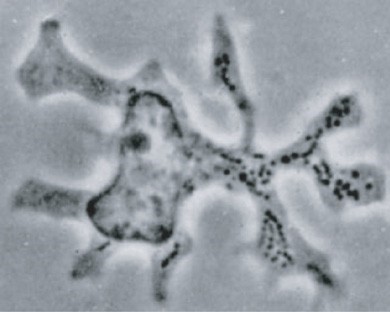
Steinman worked from the beginning of the 1970’ies with an aim to identify cells and mechanisms that could activate T cells of the adaptive immune system to carry out their many functions in directing specific immune reactions towards microorganisms as well as against other antigens. In a seminal publication from 1973 (4), he identified a cell with a dendritic (“tree like”; from the Greek word dendron) appearance in the spleen of mice (Figure 3). He later showed that this cell, which he termed the “dendritic cell”, had an exceptional capacity to activate T lymphocytes (5). His findings were not immediately accepted by the scientific community, as it was well known at the time that other cells such as macrophages were able to activate T cells against specific antigens. Through a series of ingenious experiments over several years, Steinman showed, however, that the DC had unique properties, distinct from other cells including macrophages, in being able to activate naïve T cells to specific antigens.
Importantly, he also showed that the DC is a very dynamic cell, which upon differentiation from an immature to a mature DC, induced by immune cytokines, acquires an exceptional capacity to activate and direct T cells towards specific effector functions (6). This was a key discovery, as it conceptually tied together Steinman’s research on the DC and a number of observations on so called Langerhans cells of the skin (see review in (57)), originally described by Langerhans in 1868 (58). Parallel to the work of Steinman, several groups had reported on the morphology and possible function of Langerhans cells (see review in (58)), implicating functions relating to the immune system: these cells were often observed close to lymphocytes in tissues and lymphatic vessels; they expressed MHC class II molecules; they appeared to bind to contact allergens, and they could stimulate T cells in mixed lymphocyte reactions (MLR) (59‐62). Steinman demonstrated that the latter capacity was weak, but that Langerhans cells acquired all the properties of DCs upon maturation induced by cytokines, including an exceptional capacity to activate and direct T cells towards specific effector functions (6). This was a key discovery, as it conceptually tied together Steinman’s research on the DC and a number of observations on Langerhans cells. This paved the way for the current paradigm, which states that immature DC (where Langerhans cells are considered one specialized subset of DC) patrol peripheral tissues to take up antigen, can be induced by a variety of stimuli to migrate to lymphoid organs, and concurrently develop a phenotype with all the cell surface molecules required for triggering naïve T cells. DC, with their distribution in almost all organs of the body, thus provide the first cellular connection to cells of the adaptive immune system, directing much of the subsequent T and B cell mediated immunity.
Further work by Steinman and others on the dynamics of the DC demonstrated that this cell not only had an exceptional capacity to activate adaptive immunity, but that it could also induce subsets of T cells that can prevent or down regulate immunity (63,64). This latter finding has profound implications for the understanding of how the adaptive immune system is regulated to attack foreign intruders, while tolerating normal autologous cells.
The discovery of the DC and its functions by Steinman has opened up a large field of research. Methodological development in the Steinman group has been critical for this progress, as they have taught us how DCs can be isolated, cultured and propagated in large numbers, and how they can be directed towards performing several different functions (65,66). Interestingly, the discoveries concerning the role of Toll‐like receptors in innate immunity rapidly led to the recognition by several groups that signals mediated via these receptors can determine precisely which functions will be executed by the DC. Thus, the DC is a cell that creates a link between the innate and adaptive immune systems (56).
The discoveries concerning the crucial roles of the DC in directing adaptive immunity have already resulted in a series of clinical developments (66‐68). These insights are used to create better vaccines towards infections. They have also been used to construct various forms of therapeutic vaccines against cancer. The fact that the DC can also be turned into a cell that directs down‐regulation of specific immunity provides a basis for several ongoing studies in mice as well as in man, aimed at specifically down‐regulate immune reactions that contribute to chronic inflammatory diseases.
Potential future effects of the Nobel Prize‐awarded discoveries in clinical medicine
The discoveries awarded the 2011 Nobel Prize in Physiology or Medicine has provided a new understanding of how both the first (innate) and second (adaptive) lines of immune defense are activated. Expected clinical outcomes include, among other things, improved vaccines. Ongoing work in this field is expected to improve not only vaccination against microbes but also vaccinations aimed at triggering and enhancing immune reactions against tumors (66,67). Progress in this area depends both on a better use of adjuvants to enhance innate immunity though stimulation of Toll‐like receptors and other similar receptors, and on methods for turning DCs into efficient antigen‐presenting cells (66). Additional clinical progress is hoped for within the area of autoimmune and inflammatory diseases. Here, promising results have been achieved in animal models of inflammatory diseases both by interfering with innate immunity through TLR blockade (69), and by down‐regulating disease‐associated adaptive immune responses through DC manipulation (66).
Taken together, the discoveries of Bruce A. Beutler, Jules A. Hoffmann and Ralph M. Steinman have brought us closer to the goal of treating and preventing infections, cancer and inflammatory diseases by mobilizing and regulating innate and adaptive immunity.
Hans‐Gustaf Ljunggren
Professor of Infectious Diseases, Karolinska Institutet, Stockholm
Member of the Nobel Assembly
Annika Scheynius
Professor of Clinical Allergy Research, Karolinska Institutet, Stockholm
Member of the Nobel Assembly
Lars Klareskog
Professor of Rheumatology, Karolinska Institutet, Stockholm
Chairman of the Nobel Assembly
Cited literature
1. www.nobelprize.org
2. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996, 86:973‐983
3. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Riccardi‐Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in the Tlr4 gene. Science 1998, 280288:2085‐
4. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973, 137:1142‐1162
5. Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA. 1978, 75‐5132‐5136
6. Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985, 161:526‐546
7. Boman HG, Nilsson I, Rasmusson B. Inducible antibacterial defence system in Drosophila. Nature. 1972, 237:232‐235
8. Steiner H, Hultmark D, Engström A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. 1981, 292:246‐248
9. Boman HG, Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995, 13:61–92
10. Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993, 9:178‐183
11. Hoffmann JA. Innate immunity to insects. Curr Op Immunol. 1995, 7:4‐10
12. Janeway CA. Approaching the Asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989, 54:1‐13
13. Nüsslein‐Volhard C, Lohs‐Schardin M, Sander K, Cremer C. A dorso‐ventral shift of embryonic primordia in a new maternal‐effect mutant of Drosophila. Nature. 1980, 283:474‐ 766
14. Gay NJ and Keith FJ. Drosophila Toll and IL‐1 receptor. Nature. 1991, 351:355‐356
15. Sun SC, Åsling B, Faye I. Organization and expression of the immunoresponsive lysozyme gene in the giant silk moth, Hyalophora cecropia. J Biol Chem. 1991, 266:6644‐6649
16. Sun SC, Åsling B, Lee JY, Faye I. Structure and expression of the attacin genes in Hyalophora cecropia. Eur J Biochem. 1991, 196:247‐254
17. Engström Y, Kadalayil L, Sun SC, Samakovlis C, Hultmark D, Faye I. kB‐like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993, 232:327‐333
18. Kappler C, Meister M, Lagueux M, Gateff E, Hoffmann JA, Reichard JM. Insect immunity: two 17 bp repeats nesting a kB‐related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria challenged Drosophila. EMBO J. 1993, 12:1561‐1568
19. Reichhard JM, Georgel P, Meister M, Lemaitre B, Kappler C, Hoffmann JA. Expression and nuclear translocation of the rel/NF‐kB‐related morphogen dorsal during the immune response in Drosophila. Cr Acad Sci (Paris). 1993, 316:1207‐1217
20. Ip TY, Reach M, Engström Y, Kadalayil L, Cai H, Gonzalez‐Crespo S, Tatei K, Levine M. Dif, a dorsal‐related gene that mediates an immune response in Drosophila. Cell. 1993, 75:753‐763
21. Whitham S, Dinesh‐Kumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin‐1 receptor. Cell. 1994, 78:1101‐1115
22. Petersen UM, Björklund G, Ip TY, Engström Y. The dorsal‐related immunity factor, Dif is a sequence‐specific transcription factor of Drosophila cecropin gene expression. EMBO J. 1995, 14:3146‐3158
23. Rosetto M, Engström Y, Baldari CT, Telford JL, Hultmark D. Signals from the IL‐1 receptor homolog, Toll, can activate an immune response in a Drosophila hemocyte cell line. Biochem Biophys Res Commun. 1995, 209:111‐116
24. Lemaitre B, Kromer‐Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A novel mutation, immune deficiency, defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995, 92:9465‐9469
25. Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart JM, Hoffmann JA. Functional analysis and regulation of nuclear import of doral during the immune response of Drosophila. EMBO J. 1995, 14:536‐545
26. Moirsato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsoventral pattern of the Drosophila embryo. Cell. 1994, 76:677‐688
27. Mizuguchi K, Parker JS, Bludell TL, Gay NJ. Getting knotted: a model for the structure and activation of Spätzle. Trends Biochem Sci. 1998, 23:239‐242
28. Gangloff M, Weber AN, Gibbard RJ, Gay NJ. Evolutionary relationships, but functional differences, between the Drosophila and human Toll‐like receptor families. Biochem Soc Trans. 2003, 31:659‐663
29. Nomura N, Miyajima N, Sazuka T, Tanaka A, Kawarabayashi Y, Sato S, Nagase T, Seiki N, Ishikawa K, Tabata S. Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001‐KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG‐1. DNA Res. 1994, 1:27‐35
30. Medzhitov R, Preston‐Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997, 388:394‐397
31. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998, 95:588‐593
32. Taguchi T, Mitcharn JL, Dower SK, Sims JE, Testa JR. Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor Toll, to human chromosome 4p14. Genomics. 1996, 32:486‐488
33. Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003, 3:169‐176
34. Westphal O, Nowotny A, Luderitz O, Hurni H, Eichenberger E, Schonholzer G. Die Bedeutung der Lipoid‐Komponente (Lipoid A) für die biologischen Wirkungen bakterieller Endotoxine (Lipopolysaccharide). Pharm Acta Helv 1958, 33:401‐411
35. Heppner G and Weiss DW. High susceptibility of strain A mice to endotoxin and endotoxin‐red blood cell mixtures. J Bacteriol. 1965, 90:696‐703
36. Sultzer BM. Genetic control of leucocyte responses to endotoxin. Nature 1968, 219:1253‐ 1254
37. Watson J and Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic responses to lipopolysaccharides. J Exp Med. 1974, 140:1147‐1161
38. Coutinho A, Möller G, Gronowicz E. Genetic control of B‐cell responses. IV. Inheritance of the unresponsiveness to lipopolysaccharides. J Exp Med. 1975, 142:253‐258
39. Coutinho A, Gronowicz E, Sultzer BM. Genetic control of B‐cell responses. I Selective unresponsiveness to lipopolysaccharide. Scand J Immunol. 1975, 4:139‐143
40. Moore RN, Goodrum KJ, Berry LJ. Mediation of an endotoxic effect by macrophages. J Reticuloendothelial Soc. 1976, 19:187‐197
41. Watson J, Riblet R, Taylor BA. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977, 118:2088‐2093
42. Rosenstreich DL, Weinblatt AC, O’Brian AD. Genetic control of resistance to infection in mice. Crit Rev Immunol. 1982, 3:263‐330
43. Freudenberg N, Freudenberg MA, Bandara K, Galanos C. Distribution and localization of endotoxin in the reticulo‐endothelial system (RES) and in the main vessels of the rat during shock. Pathol Res Pract. 1985, 179:517‐527
44. Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, Ulevitch R, Cerami A. Identity of tumor necrosis factor and the macrophage‐secreted factor cachectin. Nature 1985, 316:552‐554
45. Beutler B, Milsark IW, Cerami AC. Passive immunization against cachetin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985, 229:869‐871
46. Tobias PS, Soldau K, Ulevitch RJ. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem 1989, 264:10867‐10871
47. Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science 1990, 249:1429‐1431
48. Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosin phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem 1993, 268:25009‐25014
49. Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994, 265:808‐811
50. Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram‐negative bacteria in CD14‐deficient mice. Immunity 1996, 4:407‐414
51. Qureshi ST, Gros P, Malo D. The Lps locus: genetic regulation of host responses to bacterial lipopolysaccaride. Inflamm Res. 1999, 48:613‐620
52. Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002, 20:197‐ 216
53. Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001, 13:114‐119
54. Kawai T, Akira S. Toll‐like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011, 34:637‐650
55. Fearon DT and Locksely RM The instructive role of innate immunity in the aquired ‐‐ immune response. Science. 1996, 272:50‐53
56. Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll‐like receptors control activation of adaptive immune responses. Nat Immunol. 2001 2:947‐950
57. Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin‐expressing dendritic cell subsets in the skin. Immunol Rev. 2010, 234:120‐141
58. Langerhans P. Uber die nerven der menschlichen haut. Virchows Arch. 1868. 44:325‐337
59. Silberberg I. Apposition of mononuclear cells to Langerhans cells in contact allergic reactions. An ultrastructural study. Acta Derm Venerol. 1973, 53:1‐12
60. Silberberg‐Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen‐bearing Langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976, 25:137‐ 151
61. Klareskog L, Tjernlund U, Forsum U, Peterson PA. Epidermal Langerhans cells express Ia antigens. Nature. 1977, 268:248‐250
62. Stingl G, Katz SI, Celment L, Green I, Shevache EM. Immunologic functions of Ia‐bearing epidermal Langerhans cells. J Immunol. 1978, 121:2005‐2013
63. Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001, 194:769‐79
64. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003, 21:685‐711
65. Van Voorhis WC, Hair LS, Steinman RM, Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982, 155:1172‐87
66. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007 449:419‐ 26
67. Drake CG. Update on prostate cancer vaccines. The Cancer Journal. 2011, 17:294‐299
68. Schuler G. Dendritic cells Indispensible. The Cancer Journal. 2011, 17:337‐342
69. Keogh B, Parker AE. Toll‐like receptors as targets for immune disorders. Trends in Pharm Sci. 2011, 32:435‐442
© The Nobel Committee for Physiology or Medicine 2011
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
