Press release
English
Swedish

9 October 1996
The Royal Swedish Academy of Sciences has decided to award the 1996 Nobel Prize in Chemistry to
Professor Robert F. Curl, Jr., Rice University, Houston, USA,
Professor Sir Harold W. Kroto, University of Sussex, Brighton, U.K., and
Professor Richard E. Smalley, Rice University, Houston, USA,
for their discovery of fullerenes.
The discovery of carbon atoms bound in the form of a ball is rewarded
New forms of the element carbon – called fullerenes – in which the atoms are arranged in closed shells was discovered in 1985 by Robert F. Curl, Harold W. Kroto and Richard E. Smalley. The number of carbon atoms in the shell can vary, and for this reason numerous new carbon structures have become known. Formerly, six crystalline forms of the element carbon were known, namely two kinds of graphite, two kinds of diamond, chaoit and carbon(VI). The latter two were discovered in 1968 and 1972.
Fullerenes are formed when vaporised carbon condenses in an atmosphere of inert gas. The gaseous carbon is obtained e.g. by directing an intense pulse of laser light at a carbon surface. The released carbon atoms are mixed with a stream of helium gas and combine to form clusters of some few up to hundreds of atoms. The gas is then led into a vacuum chamber where it expands and is cooled to some degrees above absolute zero. The carbon clusters can then be analysed with mass spectrometry.
Curl, Kroto and Smalley performed this experiment together with graduate students J.R. Heath and S.C. OBrien during a period of eleven days in 1985. By fine-tuning the experiment they were able in particular to produce clusters with 60 carbon atoms and clusters with 70. Clusters of 60 carbon atoms, C60, were the most abundant. They found high stability in C60, which suggested a molecular structure of great symmetry. It was suggested that C60 could be a “truncated icosahedron cage”, a polyhedron with 20 hexagonal (6-angled) surfaces and 12 pentagonal (5-angled) surfaces. The pattern of a European football has exactly this structure, as does the geodesic dome designed by the American architect R. Buckminster Fuller for the 1967 Montreal World Exhibition. The researchers named the newly-discovered structure buckminsterfullerene after him.
The discovery of the unique structure of the C60 was published in the journal Nature and had a mixed reception – both criticism and enthusiastic acceptance. No physicist or chemist had expected that carbon would be found in such a symmetrical form other than those already known. Continuing their work during 1985-90, Curl, Kroto and Smalley obtained further evidence that the proposed structure ought to be correct. Among other things they succeeded in identifying carbon clusters that enclosed one or more metal atoms. In 1990 physicists W. Krätschmer and D.R. Huffman for the first time produced isolable quantities of C60 by causing an arc between two graphite rods to burn in a helium atmosphere and extracting the carbon condensate so formed using an organic solvent. They obtained a mixture of C60 and C70, the structures of which could be determined. This confirmed the correctness of the C60 hypothesis. The way was thus open for studying the chemical properties of C60 and other carbon clusters such as C70, C76, C78 and C84. New substances were produced from these compounds, with new and unexpected properties. An entirely new branch of chemistry developed, with consequences in such diverse areas as astrochemistry, superconductivity and materials chemistry/physics.
Background
Many widely diverse research areas coincide in the discovery of the fullerenes. Harold W. Kroto was at the time active in microwave spectroscopy, a science which thanks to the growth of radioastronomy can be used for analysing gas in space, both in stellar atmospheres and in interstellar gas clouds. Kroto was particularly interested in carbon-rich giant stars. He had discovered and investigated spectrum lines in their atmospheres and found that they could be ascribed to a kind of long-chained molecule of only carbon and nitrogen, termed cyanopolyynes. The same sort of molecules is also found in interstellar gas clouds. Kroto’s idea was that these carbon compounds had been formed in stellar atmospheres, not in clouds. He now wished to study the formation of these long-chain molecules more closely.
He got in touch with Richard E. Smalley, whose research was in cluster chemistry, an important part of chemical physics. A cluster is an aggregate of atoms or molecules, something in between microscopic particles and macroscopic particles. Smalley had designed and built a special laser-supersonic cluster beam apparatus able to vaporise almost any known material into a plasma of atoms and study the design and distribution of the clusters. His paramount interest was clusters of metal atoms, e.g. metals included in semiconductors, and he often performed these investigations together with Robert F. Curl, whose background was in microwave and infra-red spectroscopy.
Atoms form clusters
When atoms in a gas phase condense to form clusters, a series is formed where the size of the clusters varies from a few atoms to many hundreds. There are normally two size maxima visible in the distribution curve, one around small clusters and one around large. It is often found that cer-tain cluster sizes may dominate, and the number of atoms in these is termed a “magic number”, a term borrowed from nuclear physics. These dominant cluster sizes were assumed to have some special property such as high symmetry.
Fruitful contact
Through his acquaintanceship with Robert Curl, Kroto learned that it should be possible to use Smalley’s instrument to study the vaporisation and cluster formation of carbon, which might afford him evidence that the long-carbon-chain compounds could have been formed in the hot parts of stellar atmospheres. Curl conveyed this interest to Smalley and the result was that on 1 September 1985 Kroto arrived in Smalley’s laboratory to start, together with Curl and Smalley, the experiments on carbon vaporisation. In the course of the work it proved possible to influence drastically the size distribution of the carbon clusters where, predominantly, 60 appeared as a magic number but also 70 (Fig. 1). The research group now got something else to think about. Instead of long carbon chains, the idea arose that the C60 cluster could have the structure of a truncated (cut off) icosahedron (Fig. 2) since its great stability was assumed to correspond to a closed shell with a highly symmetrical structure. C60 was given a fanciful name, buckminsterfullerene, after the American architect R. Buckminster Fuller, inventor of the geodesic dome. This hectic period ended on 12 September with the despatch of a manuscript entitled C60: Buckminsterfullerene to Nature. The journal received it on 13 September and published the article on 14 November 1985.
Sensational news
For chemists the proposed structure was uniquely beautiful and satisfying. It corresponds to an aromatic, three-dimensional system in which single and double bonds alternated, and was thus of great theoretical significance. Here, moreover, was an entirely new example from a different research tradition with roots in organic chemistry: producing highly symmetrical molecules so as to study their properties. The Platonic bodies have often served as patterns, and hydrocarbons had already been synthesised as tetrahedral, cubic or dodecahedral (12-sided) structures.
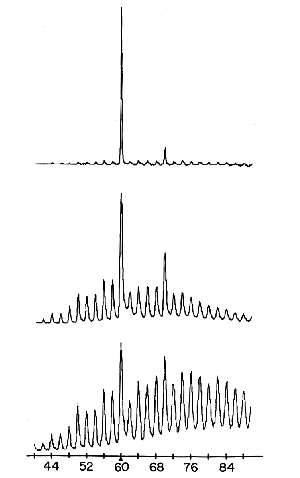
Carbon atoms per cluster
Fig. 1.
Using mass spectroscopy it was found that the size distribution of carbon clusters could be drastically affected by increasing the degree of chemical “boiling” in the inlet nozzle to the vacuum chamber. Clusters with 60 and 70 carbon atoms could be produced. (Acc. Chem. Res., Vol. 25, No. 3, 1992)
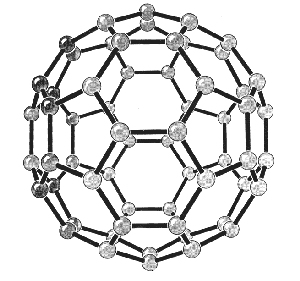 |
| Fig. 2. Models of the structures of C60. (Acc. Chem. Res., Vol. 25, No. 3, 1992) |
Further investigations
To gain further clarity Curl, Kroto and Smalley continued their investigations of C60. They attempted to make it react with other compounds. Gases such as hydrogen, nitrous oxide, carbon monoxide, sulphur dioxide, oxygen or ammonia were injected into the gas stream, but no effect on the C60 peak recorded in the mass spectrometer could be demonstrated. This showed that C60 was a slow-reacting compound. It also turned out that all carbon clusters with an even number of carbon atoms from 40-80 (the interval that could be studied) reacted equally slowly. Analogously with C60 all these should then correspond to entirely closed structures, resembling cages. This was in agreement with Euler’s law, a mathematical proposition which states that for any polygon with n edges, where n is an even number greater than 22, at least one polyhedron can be constructed with 12 pentagons and (n-20)/2 hexagons, which, in simple terms, states that it is possible with 12 pentagons and with none or more than one hexagon to construct a polyhedron. For large n many different closed structures can occur, thus also for C60, and it was presumably the beautiful symmetry of the proposed structure that gave it the preference.
The combination of chemical inertia in clusters with even numbers of carbon atoms and the possibility that all these could possess closed structures in accordance with Euler’s law, led to the proposal that all these carbon clusters should have closed structures. They were given the name fullerenes and conceivably an almost infinite number of fullerenes could exist. The element carbon had thus assumed an almost infinite number of different structures.
C60 and metals
New experiments were rapidly devised to test the C60 hypothesis. Since the C60 structure is hollow, with room for one or more other atoms, attempts were made to enclose a metal atom. A graphite sheet was soaked with a solution of a metal salt (lanthanum chloride, LaCl3) and subjected to vaporisation-condensation experiments. Masspectroscopic analysis of the clusters formed showed the presence of C60La+. These proved to be photoresistant, i.e. irradiation with intense laser light did not remove the metal atoms. This reinforced the idea that metal atoms were captured inside the cage structure.
The possibility of producing clusters with a metal atom enclosed gave rise to what was termed the “shrink-wrapping” experiment. Ions of one and the same size or at least similar sizes were gathered in a magnetic trap and subjected to a laser pulse. It then turned out that the laser beam caused the carbon cage to shrink by 2 carbon atoms at a time: at a certain cage size, when the pressure on the metal atom inside became too great, the fragmentation ceased. The shell had then shrunk so that it fitted exactly around the metal atom. For C60Cs+ this size was at C48Cs+, for C60K+ it was at C44K+ and for C60+ at C32+.
Further strong evidence gave rise to new chemistry
At the end of the 1980s, strong evidence was available that the C60 hypothesis was correct. In 1990 the synthesis of macroscopic quantities of C60 through carbon arc vaporisation between two graphite electrodes permitted the attainment of full certainty – the whole battery of methods for structure determination could be applied to C60 and other fullerenes and completely confirmed the fullerene hypothesis. As opposed to the other forms of carbon the fullerenes represent well-defined chemical compounds with in some respects new properties. A whole new chemistry has developed to manipulate the fullerene structure, and the properties of fullerenes can be studied systematically. It is possible to produce superconducting salts of C60, new three-dimensional polymers, new catalysts, new materials and electrical and optical properties, sensors, and so on. In addition, it has been possible to produce thin tubes with closed ends, nanotubes, arranged in the same way as fullerenes. From a theoretical viewpoint, the discovery of the fullerenes has influenced our conception of such widely separated scientific problems as the galactic carbon cycle and classical aromaticity, a keystone of theoretical chemistry. No practically useful applications have yet been produced, but this is not to be expected as early as six years after macroscopic quantities of fullerenes became available.
Further reading
Jim Baggott, Perfect Symmetry: The Accidental Discovery of Buckminsterfullerene, Oxford University Press, 1994, IX + 315 pp.
Hugh Aldersey-Williams, The Most Beautiful Molecule: An Adventure in Chemistry, Aurum Press, London, 1995, IX + 340 pp.
Robert F. Curl and Richard E. Smalley, Probing C60, Science, 18 Nov. 1988 Vol. 242.
Harold Kroto, Space, Stars, C60, and Soot, Science, 25 Nov. 1988 Vol. 242.
H.W. Kroto, A.W. Allaf, and S.P. Balm, C60: Buckminsterfullerene, American Chemical Society, 1991.
Richard E. Smalley, Great Balls of Carbon; The Story of Buckminsterfullerene, The Sciences, March/April 1991.
The All-Star of Buckyball; Profile: Richard E. Smalley, Scientific American, September 1993.
Rudy M. Baum, Commercial Uses of Fullerenes and Derivatives Slow to Develop, News Focus, Nov. 22, 1993 C&EN.
Hargittai, Istv(SIGMA)n, Discoverers of Buckminsterfullerene, The Chemical Intelligencer, Springer-Verlag, New York, 1995.
Robert F. Curl Jr., was born in 1933 in Alice, Texas, USA: Ph.D. in chemistry in 1957 at University of California, Berkeley, USA. Curl has since 1958 worked at Rice University, where he has been a professor since 1967.
Professor Robert F. Curl Jr.
Department of Chemistry
Rice University
P.O. Box 1892
Houston, TX 77251, USA
Sir Harold W. Kroto was born in 1939 in Wisbech, Cambridgeshire, UK. He obtained his Ph.D. in 1964 at the University of Sheffield, UK. In 1967 he moved to the University of Sussex, where he still works. In 1985 he became Professor of Chemistry there and in 1991 Royal Society Research Professor.
Professor Sir Harold W. Kroto
School of Chemistry and Molecular Sciences
University of Sussex
Brighton, Sussex BN1 9QJ, UK
Richard E. Smalley was born in 1943 in Akron, Ohio, USA. Ph.D. in chemistry 1973 at Princeton University, USA. Professor of Chemistry at Rice University since 1981 and also Professor of Physics at the same university since 1990. Member of the National Academy of Sciences in the USA and other bodies.
Professor Richard E. Smalley
Department of Chemistry
Rice University
P.O. Box 1892
Houston, TX 77251, USA
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
